Pramipexole is a dopamine agonist medication commonly prescribed for the treatment of Parkinson’s disease and restless legs syndrome (RLS). It works by stimulating dopamine receptors in the brain to help improve motor control and reduce symptoms. Maintaining the pharmaceutical purity of Pramipexole is essential to ensure patient safety, therapeutic effectiveness, and adherence to regulatory standards. Impurity profiling is important to detect and manage any harmful by-products that may arise during manufacturing, storage, or degradation processes.
Showing 1 - 5 of 5 products
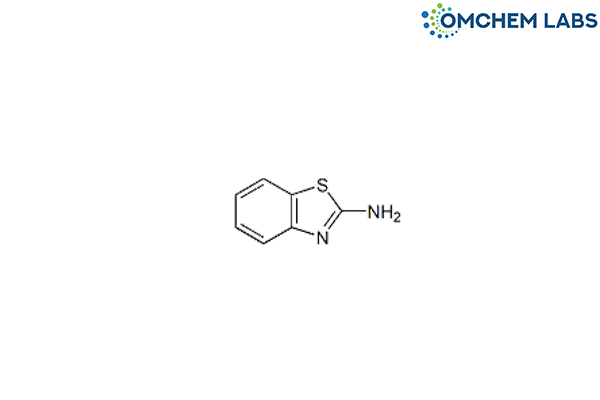
Catalogue No : PRAM-OCL-001
CAS No : 136-95-8
In Stock
Synonyms
Pramipexole BTA Impurity Benzo[d]thiazol-2-amine 2-Aminobenzothiazole
Pramipexole BTA Impurity Benzo[d]thiazol-2-amine 2-Aminobenzothiazole
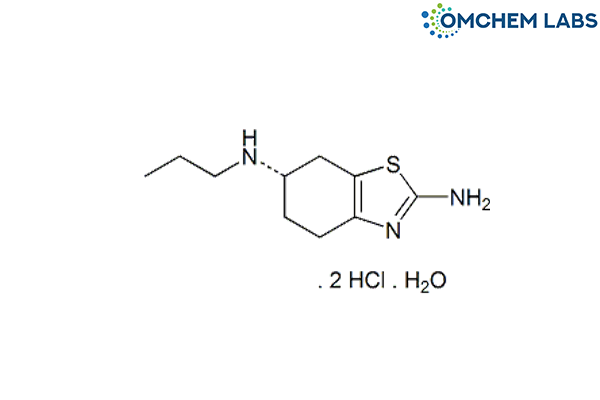
Catalogue No : PRAM-OCL-002
CAS No : 191217-81-9
In Stock
Synonyms
Pramipexole Dihydrochloride Monohydrate (S)-2-Amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole dihydrochloride monohydrate
Pramipexole Dihydrochloride Monohydrate (S)-2-Amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole dihydrochloride monohydrate
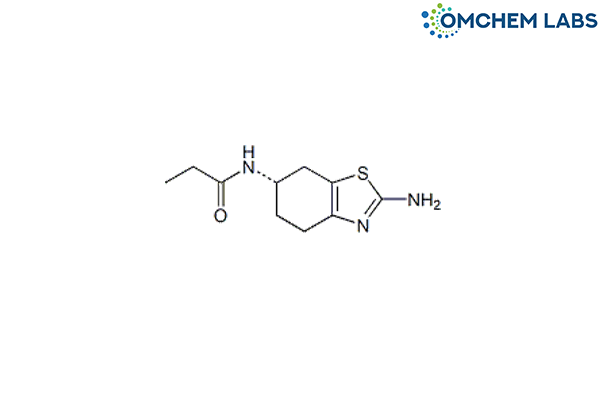
Catalogue No : PRAM-OCL-003
CAS No : 106006-84-2
In Stock
Synonyms
Pramipexole BP Impurity E Pramipexole Propionamide (USP) (S)-2-Amino-4,5,6,7-tetrahydro-6-(1-oxo-propylamino)benzothiazole
Pramipexole BP Impurity E Pramipexole Propionamide (USP) (S)-2-Amino-4,5,6,7-tetrahydro-6-(1-oxo-propylamino)benzothiazole
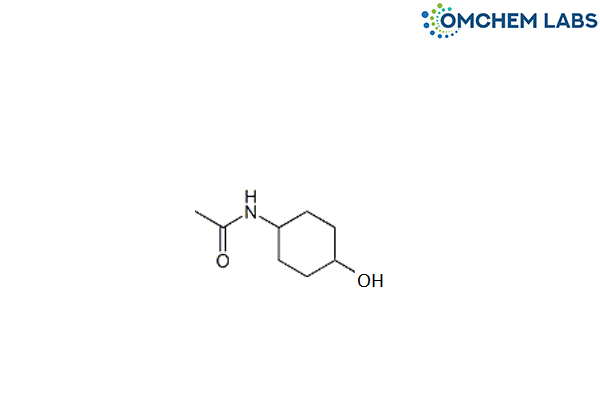
Catalogue No : PRAM-OCL-004
CAS No : 23363-88-4
In Stock
Synonyms
N-(4-Hydroxycyclohexyl)acetamide
N-(4-Hydroxycyclohexyl)acetamide
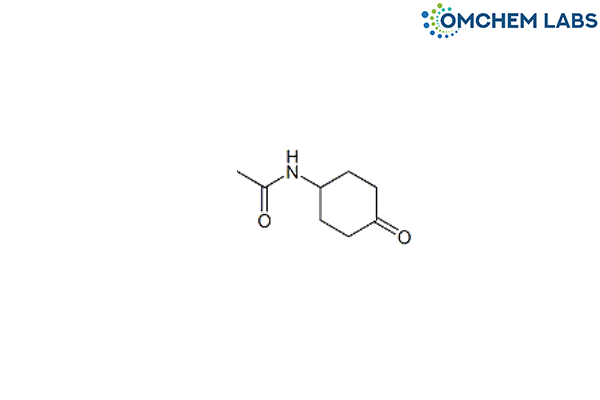
Catalogue No : PRAM-OCL-005
CAS No : 27514-08-5
In Stock
Synonyms
N-(4-Oxocyclohexyl)acetamide
N-(4-Oxocyclohexyl)acetamide
