Repaglinide is an oral antidiabetic medication primarily used to manage type 2 diabetes by stimulating insulin release from the pancreas. Unlike some other diabetes drugs, Repaglinide acts quickly and is taken before meals to control postprandial blood glucose levels. Maintaining pharmaceutical purity of Repaglinide is essential to ensure patient safety, effective glycemic control, and adherence to regulatory standards. Impurity profiling helps detect and manage potentially harmful substances formed during its manufacturing, storage, or degradation.
Showing 1 - 4 of 4 products
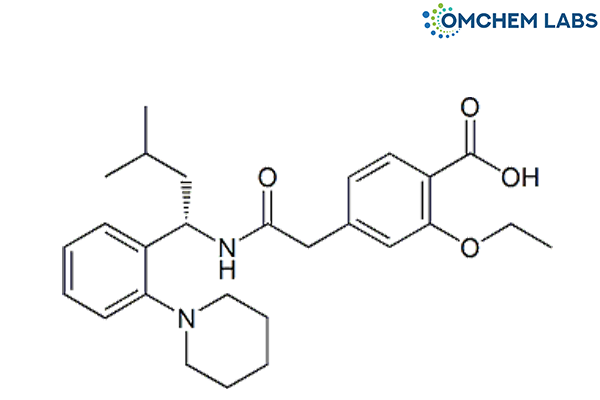
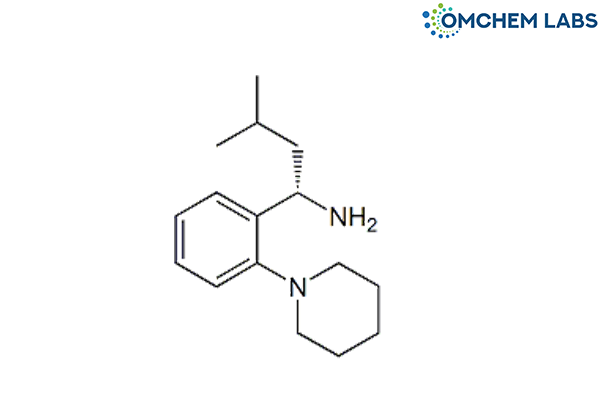
Catalogue No : REPA-OCL-001
CAS No : 135062-02-1
In Stock
Synonyms
Repaglinide 2-Ethoxy-4-[2-[[(1S)-3-methyl-1-[2-(piperidin-1-yl)phenyl] butyl] amino]-2-oxoethyl]benzoic acid
Repaglinide 2-Ethoxy-4-[2-[[(1S)-3-methyl-1-[2-(piperidin-1-yl)phenyl] butyl] amino]-2-oxoethyl]benzoic acid
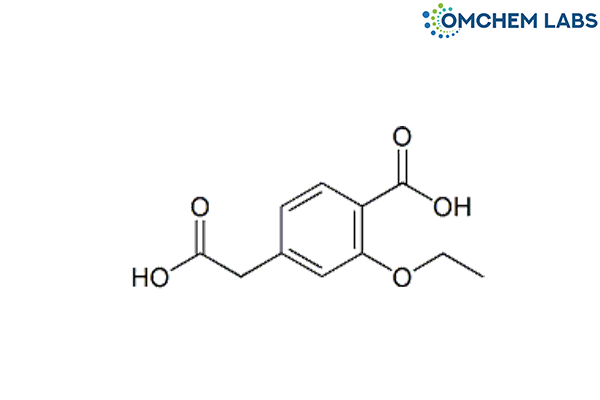
Catalogue No : REPA-OCL-002
CAS No : 220438-80-2
In Stock
Synonyms
Repaglinide BP Impurity A 4-(Carboxymethyl)-2-ethoxybenzoic acid
Repaglinide BP Impurity A 4-(Carboxymethyl)-2-ethoxybenzoic acid
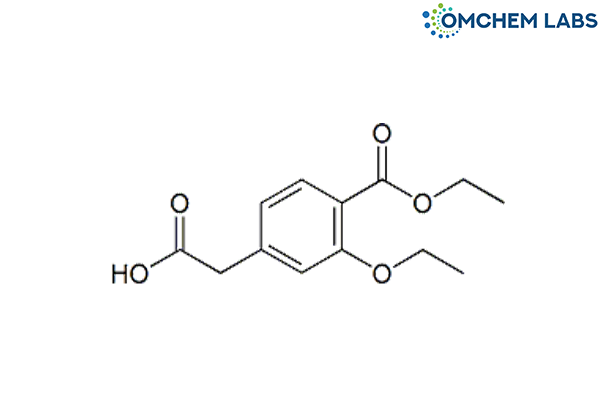
Catalogue No : REPA-OCL-003
CAS No : 99469-99-5
In Stock
Synonyms
Repaglinide BP Impurity B Repaglinide USP RC B [3-Ethoxy-4-(ethoxycarbonyl)phenyl]acetic acid
Repaglinide BP Impurity B Repaglinide USP RC B [3-Ethoxy-4-(ethoxycarbonyl)phenyl]acetic acid