Carbamazepine is an anticonvulsant and mood-stabilizing medication commonly used in the treatment of epilepsy, trigeminal neuralgia, and bipolar disorder. It functions by reducing excessive nerve signals in the brain, primarily through sodium channel blockade. Maintaining the pharmaceutical purity of Carbamazepine is essential to ensure its safety, effectiveness, and adherence to regulatory standards. Impurity profiling is vital for detecting and controlling unwanted substances that may arise during manufacturing, storage, or degradation. Here are some of its known impurities listed below.
Showing 1 - 2 of 2 products
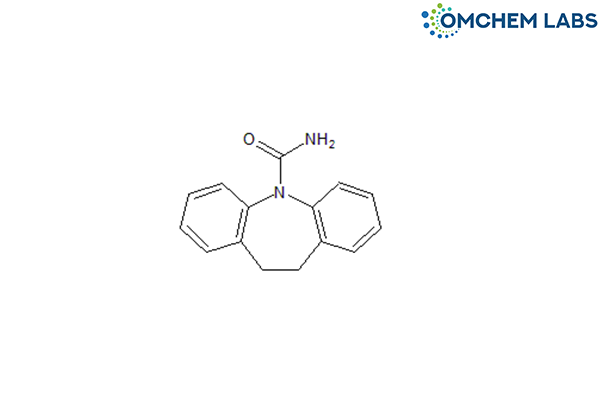
Catalogue No : CAMA-OCL-001
CAS No : 3564-73-6
In Stock
Synonyms
Carbamazepine EP Impurity A Carbamazepine USP RC A 10,11-Dihydro-5H-dibenzo[b,f]azepine-5-carboxamide 10,11-Dihydro carbamazepine
Carbamazepine EP Impurity A Carbamazepine USP RC A 10,11-Dihydro-5H-dibenzo[b,f]azepine-5-carboxamide 10,11-Dihydro carbamazepine
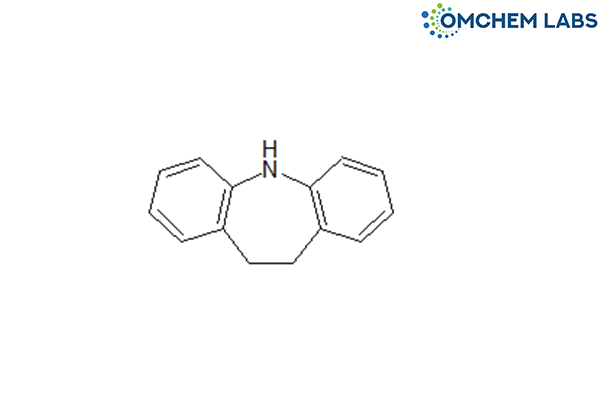
Catalogue No : CAMA-OCL-002
CAS No : 494-19-9
In Stock
Synonyms
Carbamazepine EP Impurity E 10,11-Dihydro-5H-dibenzo[b,f]azepin Iminodibenzyl
Carbamazepine EP Impurity E 10,11-Dihydro-5H-dibenzo[b,f]azepin Iminodibenzyl
