Lamivudine is an antiviral medication widely used under various brand names for the treatment of HIV-1 infection and chronic hepatitis B. As a nucleoside reverse transcriptase inhibitor (NRTI), Lamivudine works by blocking the action of reverse transcriptase, an enzyme essential for viral replication. Maintaining the pharmaceutical purity of Lamivudine is essential to ensure its therapeutic effectiveness, patient safety, and adherence to regulatory standards. Impurity profiling is crucial for detecting and controlling unwanted by-products that may arise during synthesis, storage, or degradation. Here are some of its known impurities listed below.
Showing 1 - 3 of 3 products
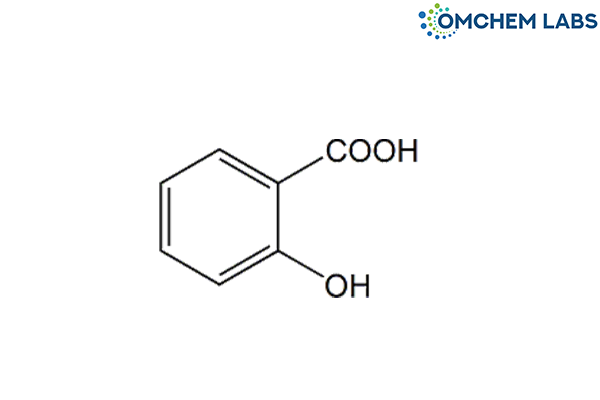
Catalogue No : LAMI-OCL-001
CAS No : 69-72-7.
Synonyms
Lamivudine EP Impurity C Salicylic Acid 2-Hydroxybenzenecarboxylic acid
Lamivudine EP Impurity C Salicylic Acid 2-Hydroxybenzenecarboxylic acid
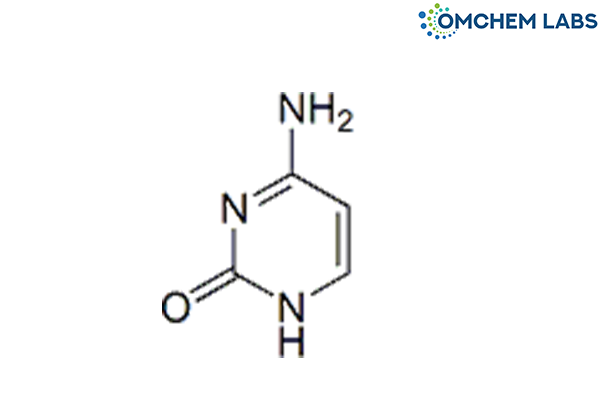
Catalogue No : LAMI-OCL-003
CAS No : 71-30-7
In Stock
Synonyms
Lamivudine EP Impurity E Cytosine 4-Aminopyrimidin-2(1H)-one
Lamivudine EP Impurity E Cytosine 4-Aminopyrimidin-2(1H)-one
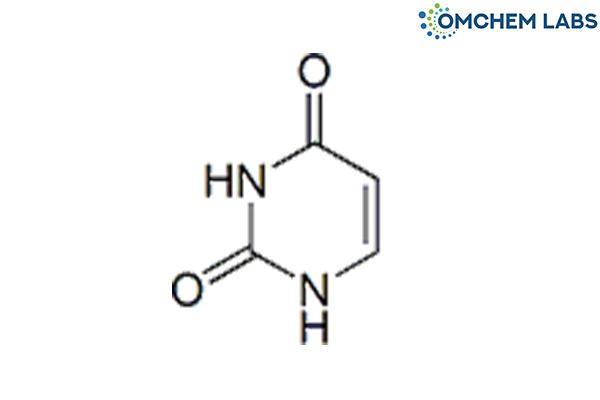
Catalogue No : LAMI-OCL-002
CAS No : 66-22-8
In Stock
Synonyms
Lamivudine EP Impurity F Uracil Pyrimidine-2,4(1H,3H)-dione
Lamivudine EP Impurity F Uracil Pyrimidine-2,4(1H,3H)-dione
