Ondansetron is a medication primarily used to prevent nausea and vomiting caused by chemotherapy, radiation therapy, or surgery. Commonly marketed under brand names like Zofran, it works by blocking serotonin receptors in the brain and gut, which helps reduce the sensation of nausea. Ensuring the pharmaceutical purity of Ondansetron is essential for patient safety, effective symptom control, and regulatory compliance. Impurity profiling plays a key role in detecting and managing any harmful by-products that may arise during manufacturing, storage, or degradation.
Showing 1 - 3 of 3 products
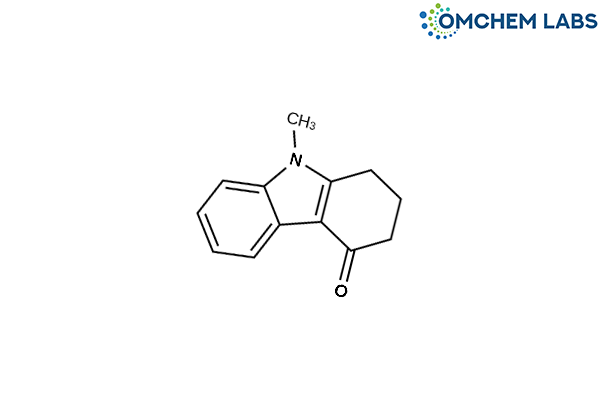
Catalogue No : ONDA-OCL-002
CAS No : 27387-31-1
In Stock
Synonyms
Ondansetron BP Impurity C Ondansetron USP Related Compound C 9-Methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one
Ondansetron BP Impurity C Ondansetron USP Related Compound C 9-Methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one
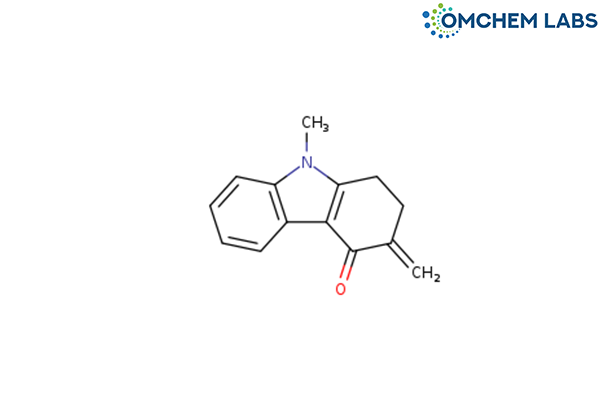
Catalogue No : ONDA-OCL-004
CAS No : 99614-64-9
In Stock
Synonyms
Ondansetron BP Impurity D Ondansetron USP Related Compound D 9-Methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one
Ondansetron BP Impurity D Ondansetron USP Related Compound D 9-Methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one
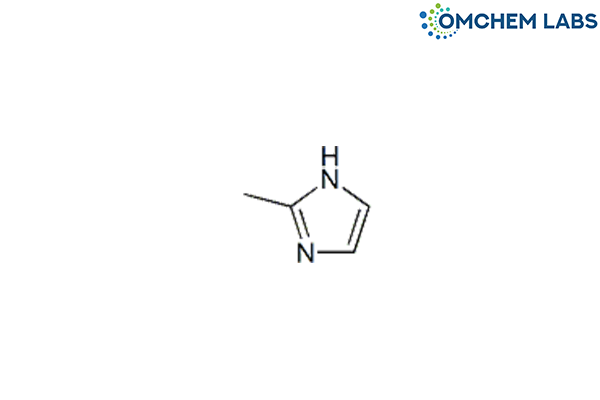
Catalogue No : ONDA-OCL-001
CAS No : 693-98-1
In Stock
Synonyms
Ondansetron BP Impurity F 2-Methyl-1H-imidazole
Ondansetron BP Impurity F 2-Methyl-1H-imidazole
