Risedronate is a bisphosphonate medication widely used to treat and prevent osteoporosis and other bone-related conditions by inhibiting bone resorption. It helps increase bone density and reduce fracture risk. Ensuring the pharmaceutical purity of Risedronate is essential for its safety, effectiveness, and regulatory adherence. Impurity profiling is important to detect and manage any contaminants or degradation products formed during manufacturing and storage to maintain therapeutic quality.
Showing 1 - 1 of 1 products
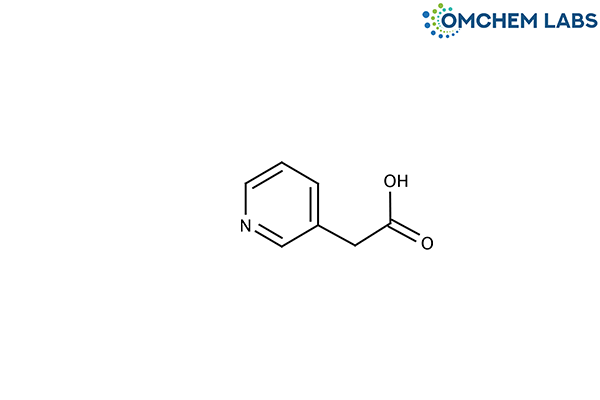
Catalogue No : RISE-OCL-001
CAS No : 501-81-5
In Stock
Synonyms
3-Pyridineacetic Acid HCl Lioxone HCl Lessterol HCl 2-(Pyridin-3-yl)acetic acid HCl
3-Pyridineacetic Acid HCl Lioxone HCl Lessterol HCl 2-(Pyridin-3-yl)acetic acid HCl
