Hydrocortisone is a corticosteroid medication widely used for its anti-inflammatory and immunosuppressive properties in the treatment of conditions such as adrenal insufficiency, allergic reactions, and various skin disorders. It mimics the natural hormone cortisol and works by reducing inflammation and modifying the body’s immune response. Ensuring the pharmaceutical purity of Hydrocortisone is essential to maintain therapeutic effectiveness, minimize adverse reactions, and meet regulatory standards. Impurity profiling is crucial to detect and control degradation products or residual impurities that may arise during manufacturing or storage. Here are some of its known impurities listed below.
Showing 1 - 1 of 1 products
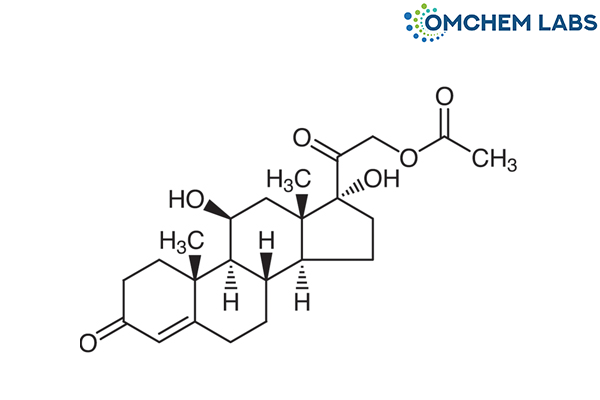
Catalogue No : HYDC-OCL-001
CAS No : 50-03-3
In Stock
Synonyms
21-Acetoxy-11β,17-dihydroxypregn-4-ene-3,20-dione Cortisol Acetate 17-Hydroxycorticosterone 21-Acetate Abbocort Bambicort Colifoam Lanacort NSC 741 P...More Detail
21-Acetoxy-11β,17-dihydroxypregn-4-ene-3,20-dione Cortisol Acetate 17-Hydroxycorticosterone 21-Acetate Abbocort Bambicort Colifoam Lanacort NSC 741 P...More Detail
