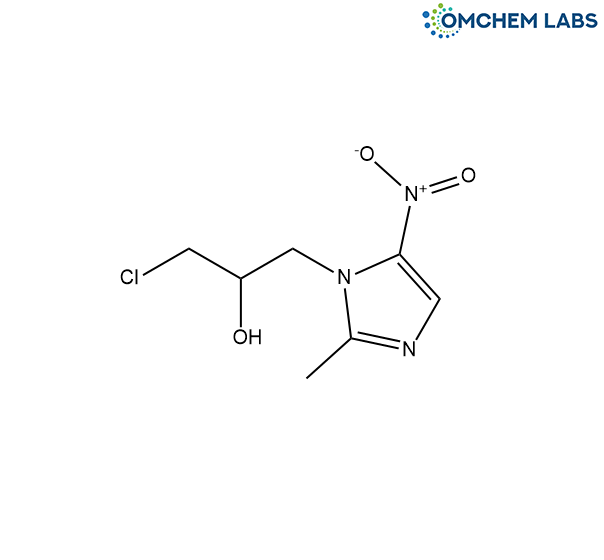
Ornidazole is an antimicrobial medication widely used to treat infections caused by anaerobic bacteria and protozoa, including conditions like amoebiasis and trichomoniasis. It works by disrupting the DNA synthesis of these microorganisms, leading to their elimination. Maintaining the pharmaceutical purity of Ornidazole is essential to ensure its safety, effectiveness, and adherence to regulatory standards. Impurity profiling is important to detect and control any potentially harmful contaminants that may arise during its manufacturing or storage processes.
Showing 1 - 1 of 1 products