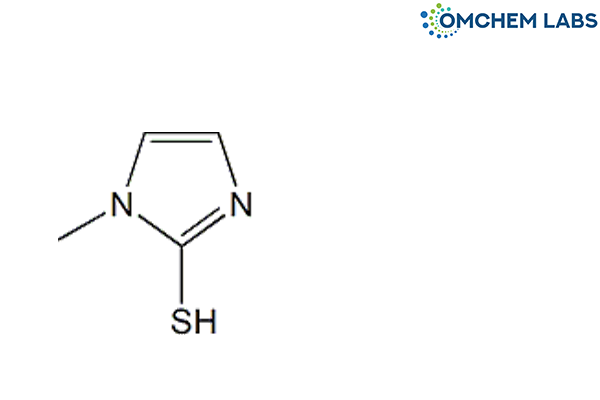
Carbimazole is an antithyroid medication primarily used in the treatment of hyperthyroidism, including Graves' disease. It works by reducing the production of thyroid hormones through inhibition of the enzyme thyroid peroxidase. Ensuring the pharmaceutical purity of Carbimazole is essential for maintaining its therapeutic effectiveness, minimizing adverse effects, and meeting regulatory standards. Impurity profiling is crucial to detect and control unwanted substances that may form during manufacturing, storage, or degradation. Here are some of its known impurities listed below.
Showing 1 - 1 of 1 products