Glipizide is an oral antidiabetic medication belonging to the sulfonylurea class, primarily used to manage type 2 diabetes mellitus by stimulating insulin release from pancreatic beta cells. It helps lower blood glucose levels and is typically prescribed when diet and exercise alone are insufficient for glycemic control. Ensuring the pharmaceutical purity of Glipizide is essential for maintaining its therapeutic effectiveness, minimizing adverse effects, and meeting regulatory standards. Impurity profiling is vital to detect and control degradation products or synthesis-related impurities. Here are some of its known impurities listed below.
Showing 1 - 5 of 5 products
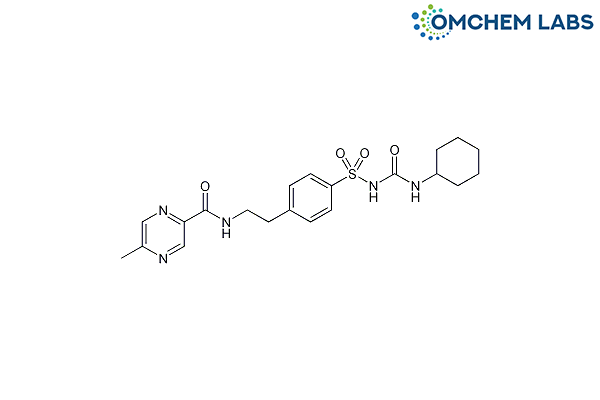
Catalogue No : GLIP-OCL-001
CAS No : 29094-61-9
In Stock
Synonyms
1-Cyclohexyl-3-[[p-[2-(5-methylpyrazinecarboxamido)ethyl]phenyl]sulfonyl]urea
1-Cyclohexyl-3-[[p-[2-(5-methylpyrazinecarboxamido)ethyl]phenyl]sulfonyl]urea
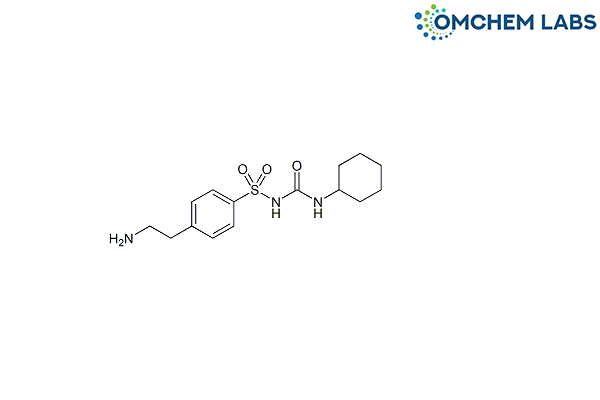
Catalogue No : GLIP-OCL-002
CAS No : 2015-16-9
In Stock
Synonyms
4-(2-Aminoethyl)-N-[(cyclohexylamino)carbonyl]benzenesulfonamide
4-(2-Aminoethyl)-N-[(cyclohexylamino)carbonyl]benzenesulfonamide
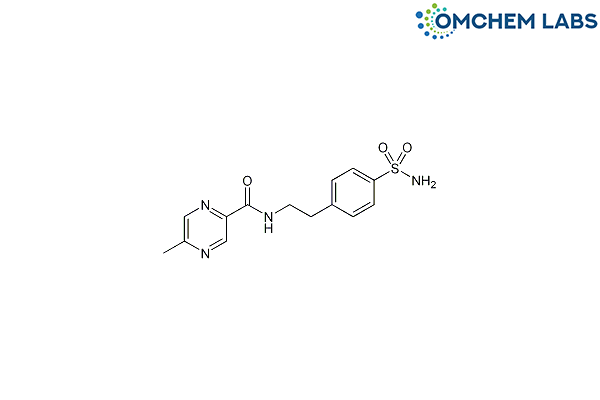
Catalogue No : GLIP-OCL-003
CAS No : 33288-71-0
In Stock
Synonyms
[2-(4-Sulphamoylphenyl) ethyl] 5-methyl-pyrazine-2-carboxamide
[2-(4-Sulphamoylphenyl) ethyl] 5-methyl-pyrazine-2-carboxamide
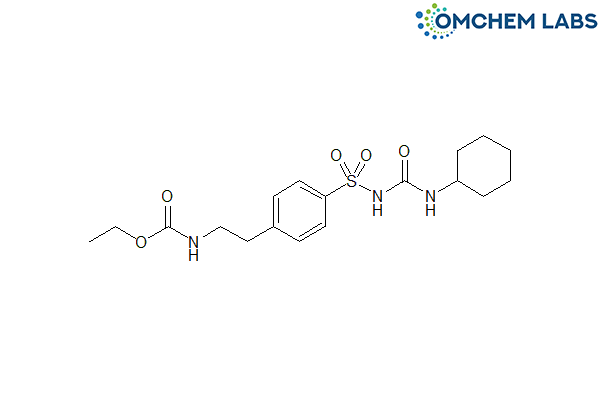
Catalogue No : GLIP-OCL-004
CAS No : 13554-93-3
In Stock
Synonyms
Ethyl 2-[4-[(cyclohexylcarbamoyl)sulphamoyl]phenyl]ethyl]carbamate
Ethyl 2-[4-[(cyclohexylcarbamoyl)sulphamoyl]phenyl]ethyl]carbamate
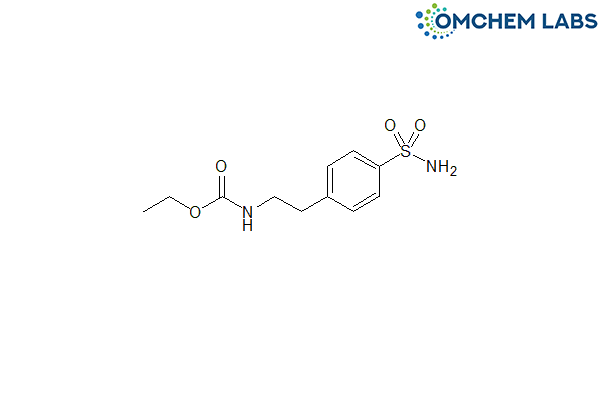
Catalogue No : GLIP-OCL-005
CAS No : 192118-08-4
In Stock
Synonyms
[2-[4-(Aminosulfonyl)phenyl]ethyl]carbamic acid ethyl ester
[2-[4-(Aminosulfonyl)phenyl]ethyl]carbamic acid ethyl ester
