Ilaprazole is a proton pump inhibitor (PPI) commonly prescribed for the treatment of gastroesophageal reflux disease (GERD), peptic ulcers, and other acid-related gastrointestinal disorders. It functions by irreversibly inhibiting the H⁺/K⁺-ATPase enzyme system in the gastric parietal cells, effectively reducing stomach acid production. Maintaining the pharmaceutical purity of Ilaprazole is essential to ensure its therapeutic effectiveness, minimize side effects, and meet stringent regulatory standards. Impurity profiling of Ilaprazole plays a crucial role in identifying and controlling degradation products or residual synthesis-related impurities that may impact drug safety and stability. Here are some of its known impurities listed below.
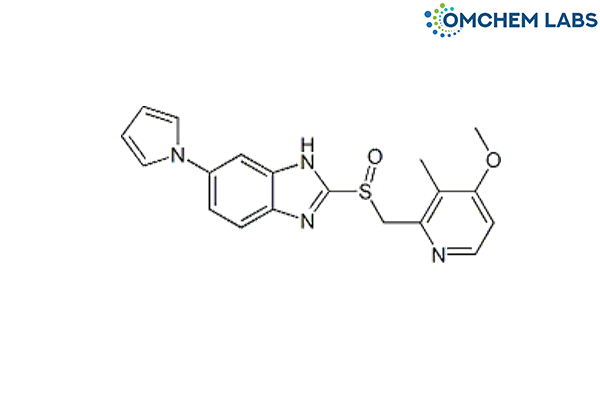
CAS No : 172152-36-2
2-((4-Methoxy-3-methylpyridin-2-yl)methylsulfinyl)-6-(1H-pyrrol-1-yl)-1H-benzo[d]imidazole
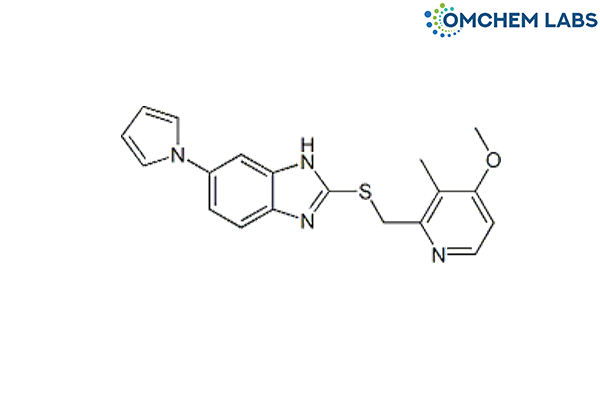
CAS No : 172152-35-1
2-((4-Methoxy-3-methylpyridin-2-yl)methylthio)-6-(1H-pyrrol-1-yl)-1H-benzo[d]imidazole
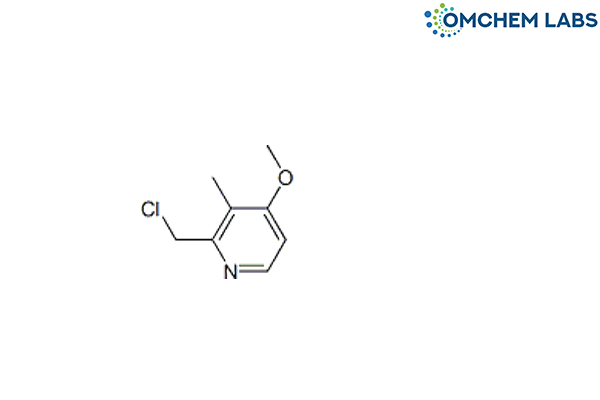
CAS No : 124473-12-7
2-(Chloromethyl)-4-methoxy-3-methylpyridine
