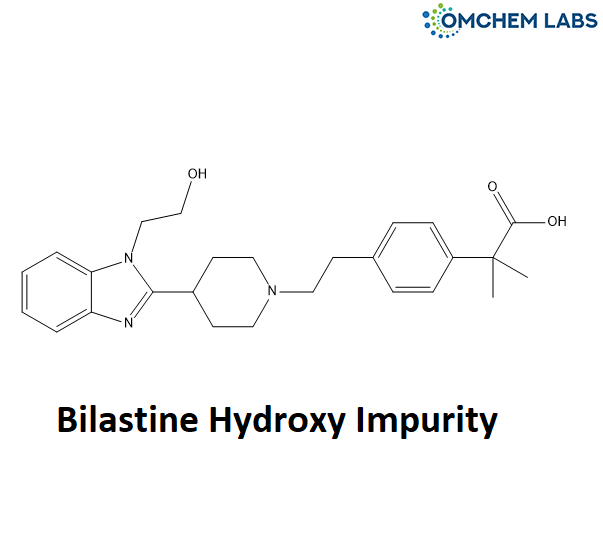
Bilastine Hydroxy Impurity
| Catalogue No |
BILA-OCL-008 |
| CAS NO |
202189-83-1 |
| Molecular Formula | C26H33N3O3 |
| Molecular weight | 435.56 |
| Inquiry Status | In Stock |
| Synonyms | 2-(4-(2-(4-(1-(2-hydroxyethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)-2-methylpropanoic acid |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Bilastine Hydroxy Impurity: A General Scientific Perspective
Introduction
Impurity profiling plays a pivotal role in ensuring the pharmaceutical integrity of drug substances. In the context of Bilastine Hydroxy Impurity, this process is critical for maintaining the therapeutic reliability and safety of the active pharmaceutical ingredient (API). As pharmaceutical regulations tighten globally, detailed understanding and control of associated impurities—particularly those arising from synthetic routes or chemical transformation—have become fundamental components of modern drug development. Profiling not only safeguards product quality but also facilitates regulatory compliance and lifecycle management.
Formation of Impurities During API Synthesis
During the multi-step synthesis of an API like Bilastine, impurities can emerge from several sources. These include incomplete reactions, side-chain interactions, over-oxidation, and reaction with trace-level contaminants or processing aids. In the specific case of Bilastine Hydroxy Impurity, such a compound could be the result of hydroxylation reactions during intermediate stages or under oxidative stress conditions. These transformations can be influenced by various synthesis parameters such as solvent environment, pH balance, temperature control, or the presence of catalysts and stabilizers. Post-synthesis handling, such as drying or packaging, may also unintentionally contribute to impurity formation through degradation or moisture-induced conversion.
Analytical Data Interpretation Techniques
A thorough impurity profile requires a multi-faceted analytical strategy. For Bilastine Hydroxy Impurity, a combination of separation and detection technologies is employed to resolve, identify, and interpret impurity signatures. Techniques such as high-performance liquid chromatography (HPLC), ultra-high-performance liquid chromatography (UHPLC), and gas chromatography (GC) are used for separation, while advanced detectors like mass spectrometry (MS) and diode array detectors (DAD) assist in structural elucidation. Nuclear magnetic resonance (NMR) spectroscopy can provide complementary confirmation for unknown impurity identities. Interpreting the analytical output involves understanding peak patterns, retention times, fragmentation behaviors, and spectral overlays—all essential for confirming impurity origins and structures without ambiguity.
Method Validation for Impurity Detection
Any analytical method applied to impurity profiling must be scientifically validated to ensure its performance and reliability. For Bilastine Hydroxy Impurity, method validation is conducted in alignment with internationally recognized guidelines to confirm that the procedures are capable of reproducibly detecting and quantifying the impurity under normal operating conditions. Validation ensures specificity for the impurity in the presence of other structural analogs, appropriate sensitivity, and robustness to minor variations in instrument or environmental conditions. This systematic validation lays the foundation for consistent quality control and regulatory submission.
Purification Strategies for Reducing Impurities
Effective impurity control extends beyond detection; it also involves the reduction or elimination of unwanted components from the final product. A tailored purification approach is applied depending on the nature and solubility of Bilastine Hydroxy Impurity. For instance, crystallization might help selectively isolate the desired API by exploiting solubility differences, while chromatography can separate closely related impurities with higher resolution. In certain cases, distillation or solvent partitioning might be useful when the impurity is volatile or exhibits differential polarity. The choice of purification technique often evolves alongside the synthetic process to balance impurity clearance with production efficiency.
Isolation and Characterization of Impurities
In scenarios where Bilastine Hydroxy Impurity is present at notable levels or remains structurally unassigned, it becomes essential to isolate and characterize it in pure form. This typically involves preparative-scale chromatography to enrich the impurity fraction, followed by detailed spectroscopic studies to determine molecular structure and identify functional groups. NMR, MS, and infrared (IR) spectroscopy collectively contribute to defining the impurity's unique chemical profile. Once characterized, the impurity can be used to generate reference standards, assess toxicological relevance, or establish regulatory thresholds, ensuring a deeper understanding of its presence and impact.
