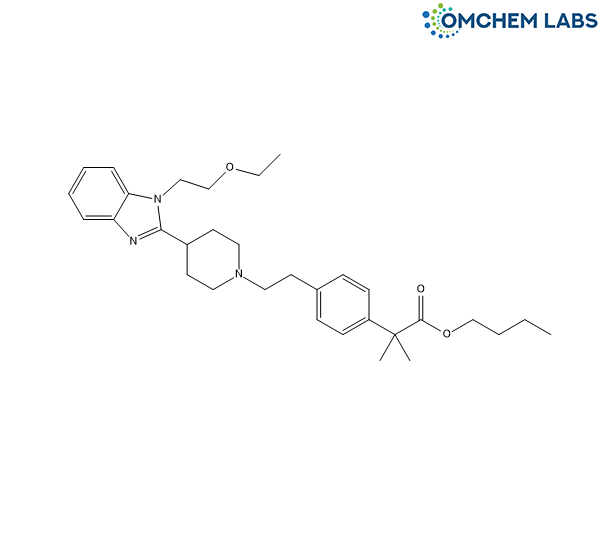
Bilastine Butyl Ester Impurity
| Catalogue No |
BILA-OCL-010 |
| CAS NO |
2699147-42-5 |
| Molecular Formula | C32H45N3O3 |
| Molecular weight | 519.70 |
| Inquiry Status | In Stock |
| Synonyms | Butyl2-(4-(2-(4-(1-(2-ethoxyethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)-2-methylpropanoate |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Bilastine Butyl Ester Impurity: A Scientific Perspective
Introduction
The study of Bilastine Butyl Ester Impurity plays a vital role in ensuring the overall safety and quality of the associated active pharmaceutical ingredient (API). Impurities, whether arising during synthesis or formed through degradation pathways, have the potential to influence pharmacological performance and patient safety. Regulatory authorities emphasize comprehensive impurity profiling as a core requirement in drug development, making it necessary to evaluate every aspect of impurity formation, detection, and control. The present overview focuses on the scientific considerations involved in assessing Bilastine Butyl Ester Impurity, highlighting strategies from synthetic understanding to analytical and purification approaches.
Formation of Impurities During API Synthesis
The occurrence of impurities in Bilastine Butyl Ester Impurity can be traced to multiple origins within the manufacturing process. Incomplete reactions, side reactions triggered by altered conditions, and the presence of reactive intermediates often give rise to unwanted molecular entities. Residual catalysts, solvents, and unreacted precursors may also persist in the final product unless carefully managed. Beyond the synthetic stage, degradation triggered by exposure to light, moisture, or temperature fluctuations further contributes to impurity formation. Recognizing these pathways is essential to designing a robust process that limits the development of extraneous materials.
Analytical Data Interpretation Techniques
The profiling of Bilastine Butyl Ester Impurity requires precise analytical tools capable of differentiating subtle structural variations. Techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and advanced mass spectrometric methods are widely employed to detect and characterize impurity signatures. Nuclear magnetic resonance (NMR) and infrared (IR) spectroscopy provide additional insight into molecular identity and bonding patterns. Interpretation of data involves correlating chromatographic peaks, mass spectral fragments, and resonance shifts to establish structural hypotheses. Such comprehensive interpretation ensures a reliable impurity profile that can be compared across different production batches.
Method Validation for Impurity Detection
Validation of analytical methods ensures that impurity detection for Bilastine Butyl Ester Impurity is both reliable and reproducible. Guidelines such as those provided by the International Council for Harmonisation (ICH) recommend testing key parameters including accuracy, precision, specificity, sensitivity, linearity, and robustness. Establishing the limit of detection and quantitation ensures that even trace amounts can be consistently identified. Without rigorous validation, analytical data may lack credibility, potentially leading to uncertainty in regulatory submissions and quality assessments.
Purification Strategies for Reducing Impurities
To minimize the presence of Bilastine Butyl Ester Impurity in the final product, purification is an indispensable stage of manufacturing. Depending on the physicochemical behavior of the impurity, strategies may include crystallization, solvent extraction, fractional distillation, or preparative chromatographic separation. Each technique leverages differences in solubility, volatility, or molecular interactions to effectively separate impurities from the desired API. Careful optimization of purification methods not only enhances purity but also preserves overall yield, making it a critical factor in large-scale production.
Isolation and Characterization of Impurities
When Bilastine Butyl Ester Impurity is present at significant levels or remains uncharacterized, isolation becomes a necessary step. Preparative-scale chromatography is often used to obtain sufficient quantities for detailed examination. Once isolated, spectroscopic techniques including NMR, MS, and IR are applied to determine the impurity’s structural identity. Characterization provides the foundation for understanding toxicological significance, enabling risk assessment and setting acceptable limits. This process also aids in building a library of impurity profiles that can be referenced in future quality evaluations.
Conclusion
The impurity profiling of Bilastine Butyl Ester Impurity illustrates the multi-faceted approach required in modern pharmaceutical science. From understanding synthetic pathways to applying advanced analytical techniques, validating methods, and employing effective purification and isolation strategies, each step contributes to building a complete impurity profile. Such profiling not only meets regulatory expectations but also supports the delivery of safe and effective medications. Ultimately, robust impurity management ensures that the final API achieves the desired standards of purity, quality, and therapeutic reliability.
