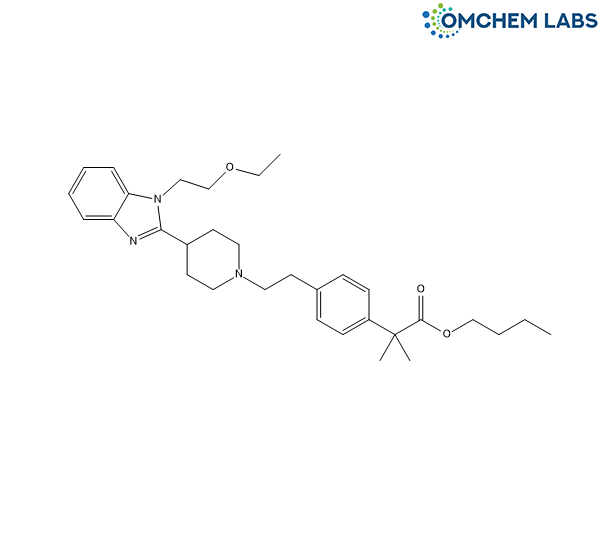
Bilastine Impurity G
| Catalogue No |
BILA-OCL-010 |
| CAS NO |
2699147-42-5 |
| Molecular Formula | C32H45N3O3 |
| Molecular weight | 519.70 |
| Inquiry Status | In Stock |
| Synonyms | Butyl2-(4-(2-(4-(1-(2-ethoxyethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)-2-methylpropanoate |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Bilastine Impurity G: A Comprehensive Scientific Overview
Introduction
Impurity profiling plays a pivotal role in the development and quality control of active pharmaceutical ingredients (APIs). In the context of Bilastine Impurity G, understanding the nature, origin, and behavior of impurities is critical to ensuring the safety, efficacy, and regulatory compliance of pharmaceutical products. Impurities, which may arise from various stages of the manufacturing process or post-synthesis degradation, can influence the pharmacological profile and patient safety. This article provides a thorough exploration of the formation, detection, and management of impurities related to Bilastine Impurity G, emphasizing strategies that maintain high standards of pharmaceutical quality.
Formation of Impurities During API Synthesis
The generation of impurities during the synthesis of Bilastine Impurity G is a multifaceted phenomenon. Impurities may be introduced as a result of incomplete reactions, side reactions, reagent residues, or interaction with environmental factors such as moisture and oxygen. The chemical pathways involved in the synthesis, including reaction conditions like temperature, pH, and reaction time, significantly impact the impurity profile. Additionally, the stability of intermediates and the susceptibility of the molecule to degradation contribute to the diversity of impurities encountered. A comprehensive understanding of these factors is essential to anticipate potential impurity formation and to develop appropriate control measures.
Analytical Data Interpretation Techniques
The accurate identification and quantification of impurities related to Bilastine Impurity G rely on sophisticated analytical methodologies. Techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), mass spectrometry (MS), and nuclear magnetic resonance (NMR) spectroscopy are routinely employed. These methods provide complementary information about the chemical identity, concentration, and structure of impurities. Interpreting chromatographic retention times, spectral fragmentation patterns, and resonance signals requires expertise to distinguish impurities from the main compound and to characterize unknown substances. This integrated analytical approach supports a robust impurity profiling framework.
Method Validation for Impurity Detection
To ensure reliability and reproducibility, analytical methods used for profiling impurities in Bilastine Impurity G must undergo stringent validation procedures. Validation parameters include specificity, sensitivity, accuracy, precision, linearity, and robustness. Conforming to international guidelines, such as those outlined by the International Council for Harmonisation (ICH), validation confirms that the analytical procedures are fit for their intended purpose. This process ensures that impurities can be consistently detected and quantified at trace levels, thus supporting product quality assurance and regulatory compliance.
Purification Strategies for Reducing Impurities
Purification is an essential step to limit the presence of impurities in the final Bilastine Impurity G product. Various purification techniques can be tailored to the physicochemical properties of the impurities and the API. Crystallization remains one of the most effective methods for separating impurities based on solubility differences. Chromatographic purification techniques, including preparative HPLC and flash chromatography, offer high resolution for separating closely related impurities. Other techniques such as solvent extraction and distillation may also be applied depending on the nature of the impurities. Selecting an appropriate purification strategy optimizes product purity and manufacturing efficiency.
Isolation and Characterization of Impurities
When impurities surpass identification or qualification thresholds, their isolation and structural elucidation become imperative. Isolation is commonly achieved through preparative chromatographic methods, enabling the collection of sufficient quantities for further study. Structural characterization is then performed using spectroscopic techniques such as NMR, MS, and infrared (IR) spectroscopy. These analyses reveal the molecular framework and potential functional groups present in the impurity. Comprehensive characterization supports toxicological evaluation and helps define impurity limits within regulatory filings, ensuring the safety of the pharmaceutical product.
Conclusion
The impurity profiling of Bilastine Impurity G encompasses a holistic approach involving the understanding of impurity formation mechanisms, application of advanced analytical techniques, rigorous method validation, effective purification strategies, and thorough structural characterization. This multi-tiered process is fundamental to safeguarding product quality, meeting regulatory standards, and ultimately protecting patient health. Establishing a proactive impurity control strategy for Bilastine Impurity G is essential for the continued development and manufacturing of high-quality pharmaceutical formulations.
