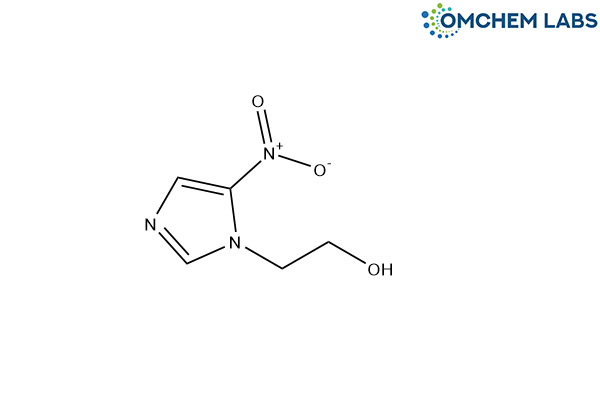
Metronidazole Impurity D
| Catalogue No |
METR-OCL-007 |
| CAS NO |
5006-68-8 |
| Molecular Formula | C5H7N3O3 |
| Molecular weight | 157.10 |
| Inquiry Status | In Stock |
| Synonyms | 2-(5-Nitro-1H-imidazol-1-yl)ethan-1-ol |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Metronidazole Impurity D: A Scientific Perspective
Introduction
Understanding the impurity landscape of pharmaceutical ingredients is a critical component of quality assurance and regulatory compliance. In the context of Metronidazole Impurity D, thorough impurity profiling supports both the safety and consistency of the active pharmaceutical ingredient (API) throughout its lifecycle. Impurities, whether inherent to the synthetic process or arising from post-synthesis events, can influence pharmacological outcomes, stability, and toxicology. Therefore, a comprehensive evaluation of such impurities is essential for establishing a robust drug substance profile and meeting global regulatory expectations.
Formation of Impurities During API Synthesis
Impurities associated with Metronidazole Impurity D often originate from multiple stages within the synthetic pathway of the API. These may arise due to incomplete reactions, undesired side reactions, reagent interactions, or the degradation of intermediates. Conditions such as prolonged reaction times, temperature variations, pH fluctuations, or solvent instability can further encourage the generation of secondary and tertiary by-products. In some cases, impurities can also result from residual starting materials or catalysts that persist through synthesis and purification stages. The formation of these unwanted entities necessitates early-stage risk assessment and process optimization to mitigate impurity buildup.
Analytical Data Interpretation Techniques
To accurately identify and monitor impurities like Metronidazole Impurity D, an integrated analytical strategy is adopted using modern instrumentation and data interpretation techniques. High-resolution chromatographic methods are employed to separate impurities from the primary API, while detection is facilitated by spectroscopic techniques such as mass spectrometry, UV spectroscopy, and nuclear magnetic resonance. These methods collectively allow for the deconvolution of complex impurity profiles. Analytical data must be carefully interpreted to distinguish between known and novel impurities, assess their structural characteristics, and trace them back to specific process variables or degradation pathways. Insightful interpretation not only enables impurity identification but also supports robust decision-making during development and scale-up.
Method Validation for Impurity Detection
Reliable impurity profiling is contingent on validated analytical methods that meet stringent performance criteria. For Metronidazole Impurity D, validation ensures the selected method is capable of detecting and quantifying impurities with consistency, selectivity, and reproducibility. Parameters such as method sensitivity, linearity, accuracy, and specificity are evaluated under diverse analytical conditions to simulate real-world scenarios. Method robustness must be proven to handle variations without compromising the integrity of results. A validated method acts as a safeguard against false positives or undetected anomalies and underpins data submitted to regulatory bodies.
Purification Strategies for Reducing Impurities
Once identified, it becomes imperative to minimize the presence of impurities such as Metronidazole Impurity D through tailored purification processes. Depending on the impurity’s chemical behavior, different techniques may be employed, including fractional crystallization, selective solvent extraction, or advanced preparative chromatography. These approaches are often optimized in alignment with the impurity’s solubility, polarity, or thermal properties. The goal is to isolate the pure API from related compounds without compromising yield or introducing additional contaminants. Effective purification also enhances downstream stability and packaging reliability by reducing impurity reformation risks.
Isolation and Characterization of Impurities
For a complete impurity profile, isolation of trace-level impurities like Metronidazole Impurity D is necessary when their structure remains unresolved or they exceed qualification thresholds. Isolation typically involves preparative chromatographic separation followed by comprehensive spectral characterization. Techniques such as NMR, IR, and high-resolution MS enable precise structural elucidation, ensuring each impurity is fully understood in terms of identity and potential impact. This step is essential not only for risk assessment but also for developing reference standards that support ongoing routine analysis and future process modifications.
Conclusion
The impurity profiling of Metronidazole Impurity D encompasses a detailed scientific workflow that integrates chemical knowledge, analytical expertise, and regulatory compliance. From recognizing how impurities form to validating detection techniques and isolating trace entities, each phase plays a vital role in ensuring drug safety and integrity. By embedding impurity profiling into the broader quality-by-design framework, pharmaceutical developers can strengthen product consistency, meet global regulatory benchmarks, and protect patient health throughout the drug’s lifecycle.
