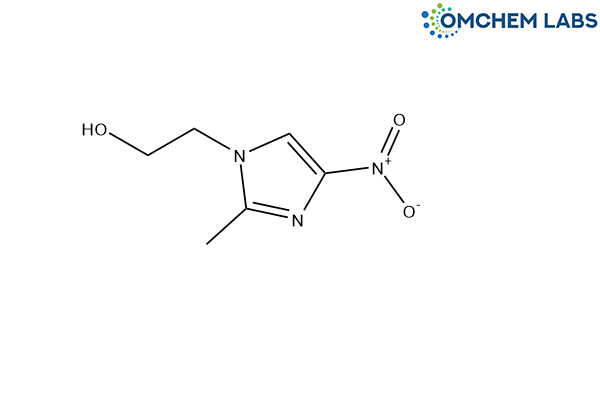
Metronidazole Impurity E
| Catalogue No |
METR-OCL-008 |
| CAS NO |
705-19-1 |
| Molecular Formula | C6H9N3O3 |
| Molecular weight | 171.15 |
| Inquiry Status | In Stock |
| Synonyms | 2-(2-methyl-4-nitro-1H-imidazol-1-yl)ethanol |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Metronidazole Impurity E: A Scientific Perspective
Introduction
The meticulous examination of impurities within pharmaceutical compounds is fundamental to drug quality and regulatory compliance. In the case of Metronidazole Impurity E, understanding its origin, behavior, and control strategies plays a critical role in ensuring the safety and effectiveness of the final active pharmaceutical ingredient (API). Impurities, if not adequately identified and managed, can compromise therapeutic performance or introduce toxicological risks. As part of an overarching pharmaceutical quality system, impurity profiling supports product development, regulatory submissions, and lifecycle control of pharmaceutical substances.
Formation of Impurities During API Synthesis
Impurities associated with Metronidazole Impurity E may emerge at multiple stages of the synthetic and post-synthetic process. Chemical reactions used in the manufacturing of the parent API often give rise to unintended by-products due to over-reactions, side reactions, or incomplete transformations. In addition, the use of specific solvents, reagents, catalysts, and intermediates introduces variables that may generate trace-level impurities. Environmental factors such as light, humidity, and oxygen exposure during storage can further induce degradation pathways. These conditions collectively contribute to the complexity of impurity profiles, necessitating robust control strategies during process design and scale-up.
Analytical Data Interpretation Techniques
Profiling of Metronidazole Impurity E requires the integration of multiple analytical techniques to accurately detect, identify, and differentiate impurities from the main compound. High-resolution chromatographic methods—such as liquid chromatography and gas chromatography—form the backbone of impurity detection. Coupled with spectral techniques like mass spectrometry (MS), nuclear magnetic resonance (NMR), and ultraviolet-visible spectroscopy (UV-Vis), these methods enable detailed structural and qualitative evaluation. The interpretation of chromatograms, spectral fingerprints, and mass fragmentation patterns offers essential insights into the presence and identity of unknown or trace impurities.
Method Validation for Impurity Detection
Ensuring the reliability of analytical data for Metronidazole Impurity E involves rigorous method validation. This process evaluates whether the analytical method performs consistently and accurately across a defined range. Parameters such as specificity, sensitivity, repeatability, linearity, and robustness are assessed to confirm the method’s fitness for purpose. A validated method not only ensures confidence in impurity detection but also meets regulatory expectations during quality control and stability testing. Validation efforts support method transferability and reproducibility across labs and production scales.
Purification Strategies for Reducing Impurities
Following detection, the next step in managing Metronidazole Impurity E involves effective removal or reduction through purification. A variety of techniques may be employed based on the physicochemical properties of both the impurity and the API. Solvent crystallization, fractional precipitation, liquid-liquid extraction, and preparative chromatography are commonly used approaches. These purification strategies aim to enhance the purity of the final product by selectively isolating undesired compounds. The efficiency of a purification method is influenced by parameters such as solubility profiles, partition coefficients, and thermodynamic stability of the compounds involved.
Isolation and Characterization of Impurities
When an impurity such as Metronidazole Impurity E exceeds reporting thresholds or lacks prior toxicological assessment, isolation and structural elucidation become imperative. Specialized chromatographic techniques, particularly preparative high-performance liquid chromatography (prep-HPLC), are used to obtain sufficient quantities of the impurity. Structural analysis is then carried out using techniques like MS, NMR, and IR spectroscopy. The goal is to determine the molecular framework and understand its potential impact on the product's safety and efficacy. Establishing structural identity is essential for qualifying the impurity and setting appropriate regulatory limits.
Conclusion
The comprehensive profiling of Metronidazole Impurity E reflects the critical role impurity control plays in pharmaceutical development. From synthesis to characterization, each phase of impurity management contributes to product safety, regulatory acceptance, and therapeutic reliability. A well-defined impurity profile supports risk-based decision-making and aligns with global regulatory standards. With evolving guidelines and increasing demand for high-purity drugs, impurity profiling remains a cornerstone of modern pharmaceutical science, enabling manufacturers to deliver safe and consistent drug products to the global market.
