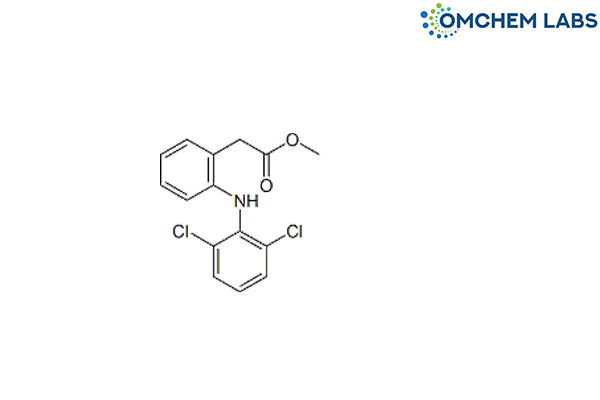
Aceclofenac Impurity B
| Catalogue No |
ACEC-OCL-003 |
| CAS NO |
15307-78-5 |
| Molecular Formula | C15H13Cl2NO2 |
| Molecular weight | 310.18 |
| Inquiry Status | In Stock |
| Synonyms | Methyl [2-[(2,6-dichlorophenyl)amino]phenyl]acetate |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
A General Overview of Aceclofenac Impurity B: Characterization and Relevance in Pharmaceutical Quality Control
Impurities in pharmaceutical compounds can influence the efficacy, safety, and regulatory compliance of drug products. Aceclofenac, a widely used non-steroidal anti-inflammatory drug (NSAID), is subject to rigorous impurity profiling to ensure quality. Among its known impurities, Aceclofenac Impurity B is a significant degradation or process-related byproduct. This paper provides a general overview of Aceclofenac Impurity B, discussing its origin, potential impact, and importance in pharmaceutical analysis and quality assurance, without delving into quantitative specifics.
1. Introduction
Aceclofenac is a commonly prescribed NSAID used for the management of pain and inflammation associated with various musculoskeletal disorders. Like many pharmaceutical substances, Aceclofenac is synthesized and stored under conditions that may lead to the formation of impurities. Regulatory guidelines emphasize the need to identify, monitor, and control such impurities to safeguard patient health and ensure therapeutic consistency. One such impurity, designated as Aceclofenac Impurity B, is noteworthy due to its presence as a degradation or synthetic byproduct.
2. Origin and Formation of Impurity B
Aceclofenac Impurity B can arise through various pathways during the synthesis, storage, or degradation of the parent drug. Common sources include incomplete reactions, side reactions, or exposure to environmental factors such as heat, moisture, and light. The chemical structure of Impurity B is typically related to Aceclofenac but with specific alterations that may affect its pharmacological or toxicological properties.
3. Significance in Pharmaceutical Quality Control
The detection and control of Impurity B are integral to maintaining the safety and efficacy profile of Aceclofenac formulations. Analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry are employed to identify and quantify this impurity. Regulatory authorities recommend or mandate thresholds for such impurities, beyond which detailed toxicological assessments may be required.
4. Regulatory and Safety Considerations
Although Impurity B may be present in trace amounts, its consistent monitoring aligns with international guidelines such as those set forth by the International Council for Harmonisation (ICH). These guidelines promote risk-based approaches to impurity control, helping ensure that pharmaceutical products meet stringent quality standards before reaching the market.
5. Conclusion
Aceclofenac Impurity B serves as an important marker in the broader context of drug purity and stability assessment. While its occurrence is not uncommon, its control remains a priority in pharmaceutical manufacturing and quality assurance. Continued vigilance in monitoring such impurities is essential for compliance, patient safety, and therapeutic reliability.
