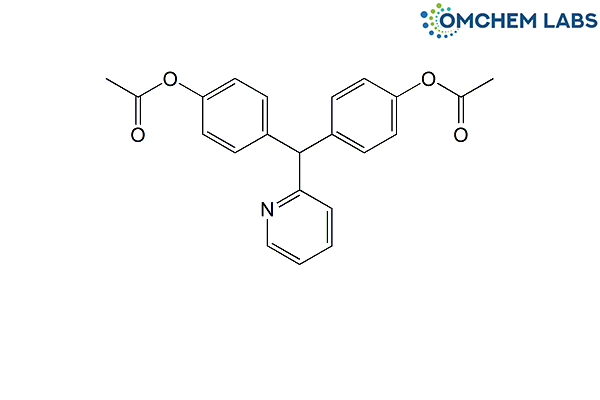
Bisacodyl
| Catalogue No |
BISA-OCL-001 |
| CAS NO |
603-50-9 |
| Molecular Formula | C22H19NO4 |
| Molecular weight | 361.39 |
| Inquiry Status | In Stock |
| Synonyms | 4,4′-(Pyridin-2-ylmethylene)diphenyl diacetate |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Bisacodyl: A Comprehensive Review
Introduction
The profiling of impurities in pharmaceutical compounds such as Bisacodyl represents a fundamental component in ensuring drug safety, efficacy, and regulatory compliance. Impurities may arise from multiple sources during manufacturing, storage, or handling, potentially impacting the quality of the active pharmaceutical ingredient (API). The accurate identification and control of these impurities are critical to maintain therapeutic integrity and meet stringent pharmacopeial standards globally. This paper provides a broad overview of the impurity formation mechanisms, analytical approaches, method validation, purification tactics, and characterization protocols relevant to Bisacodyl, with adaptability to similar compounds.
Formation of Impurities During API Synthesis
Impurity formation in Bisacodyl synthesis typically originates from inherent chemical processes, including incomplete reactions, side reactions, degradation of intermediates, and residual reagents. Reaction conditions such as temperature, pH, solvent selection, and catalyst use influence impurity profiles. Additionally, environmental factors during synthesis and subsequent storage can introduce degradation products. These impurities can be classified broadly into organic impurities, inorganic residues, and solvent traces. Understanding the synthesis pathway and conditions is essential for predicting and controlling the nature and levels of impurities.
Analytical Data Interpretation Techniques
Robust analytical techniques are imperative for comprehensive impurity profiling of Bisacodyl. Chromatographic methods such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and coupled techniques like liquid chromatography–mass spectrometry (LC-MS) enable the detection and separation of impurities based on physicochemical properties. Spectroscopic tools, including nuclear magnetic resonance (NMR) and infrared (IR) spectroscopy, provide structural insights critical for identification. The integration of chromatographic retention data with spectroscopic fingerprints allows for detailed interpretation of impurity profiles, facilitating differentiation between known impurities and unknown contaminants.
Method Validation for Impurity Detection
To guarantee reliable impurity detection in Bisacodyl, analytical methods must undergo stringent validation as outlined in guidelines such as ICH Q2(R1). Validation confirms that methods are specific, accurate, precise, robust, and sensitive enough to detect impurities at trace levels. Parameters like linearity, limit of detection (LOD), limit of quantitation (LOQ), and repeatability are evaluated to ensure the method’s suitability for routine quality control and regulatory submission. Validated methods form the backbone of impurity monitoring programs, supporting consistent product quality.
Purification Strategies for Reducing Impurities
Effective purification of Bisacodyl involves employing tailored techniques to minimize impurity levels and enhance API purity. Techniques such as recrystallization leverage differential solubility, while chromatographic purification, including preparative HPLC or flash chromatography, allows precise separation of closely related impurities. Solvent extraction and distillation may also be employed depending on impurity characteristics. Selecting an appropriate purification strategy is dependent on impurity profiles and physicochemical properties, balancing impurity removal efficiency with product yield and scalability.
Isolation and Characterization of Impurities
When impurities exceed identification thresholds or have unclear structures, isolation and characterization become essential. Preparative chromatographic methods enable separation of individual impurities for further analysis. Spectroscopic techniques including NMR, mass spectrometry (MS), and IR spectroscopy provide detailed structural elucidation. Characterization aids in assessing potential toxicological impacts and establishing impurity specifications for regulatory compliance. The creation of impurity reference standards based on isolated impurities supports ongoing analytical consistency.
Conclusion
Impurity profiling of Bisacodyl requires an integrated approach combining a deep understanding of synthetic pathways, advanced analytical techniques, rigorous validation protocols, strategic purification, and thorough impurity characterization. Such a holistic impurity management framework ensures high-quality pharmaceutical products that conform to regulatory requirements and uphold patient safety. The principles outlined herein are adaptable to related APIs and impurities, facilitating consistent quality assurance in pharmaceutical manufacturing.
