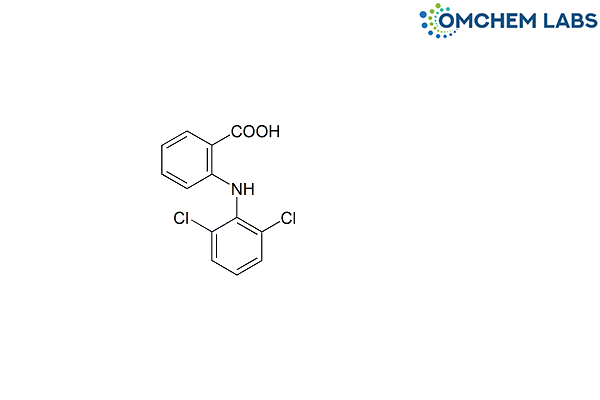
Diclofenac Carboxylic Acid
| Catalogue No |
DICL-OCL-001 |
| CAS NO |
13625-57-5 |
| Molecular Formula | C13H9Cl2NO2 |
| Molecular weight | 282.12 |
| Inquiry Status | In Stock |
| Synonyms | 2-[(2,6-Dichlorophenyl)amino]benzoic acid |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
An Overview of Diclofenac Carboxylic Acid Impurity: Formation, Significance, and Regulatory Considerations
Diclofenac is a widely used non-steroidal anti-inflammatory drug (NSAID) known for its efficacy in treating pain and inflammation. During its synthesis, storage, or metabolism, several impurities can arise, among which diclofenac carboxylic acid impurity is noteworthy. This paper provides a general overview of this impurity, focusing on its origin, significance, and regulatory implications in pharmaceutical quality control.
1. Introduction
-
Impurity profiling has become an integral aspect of pharmaceutical development, ensuring drug safety, efficacy, and regulatory compliance. Diclofenac, like many other APIs (Active Pharmaceutical Ingredients), is susceptible to forming impurities during various stages of its lifecycle. One such impurity, diclofenac carboxylic acid impurity, can be formed as a process-related or degradation product. Understanding its nature and impact is essential for maintaining pharmaceutical quality.
2. Origin and Formation
- Diclofenac carboxylic acid impurity is typically formed through hydrolysis or oxidative degradation pathways. This impurity may also arise as a by-product during the synthesis of diclofenac or under stressed storage conditions. Environmental factors such as pH, temperature, and exposure to light or oxygen can influence its formation.
3. Significance in Pharmaceutical Analysis
- While impurities are generally present at low levels, their identification and quantification are crucial. Diclofenac carboxylic acid impurity, due to its structural similarity to the parent compound, may affect the pharmacological profile or stability of the final formulation. Therefore, its presence must be monitored using validated analytical methods as part of routine quality control processes.
4. Regulatory Perspective
- Regulatory agencies such as the ICH (International Council for Harmonisation) provide guidelines on impurity thresholds and control strategies. Even though specific limits vary, the presence of diclofenac carboxylic acid impurity must be justified, controlled, and documented as part of the drug approval process. Risk assessment approaches are often applied to determine its safety margins.
5. Conclusion
- Diclofenac carboxylic acid impurity represents a common but manageable challenge in the manufacture and storage of diclofenac-based formulations. While it does not usually pose major concerns at trace levels, its identification and control remain vital to ensuring product quality and regulatory compliance. Continued research and monitoring help ensure the safety and effectiveness of pharmaceutical products.
