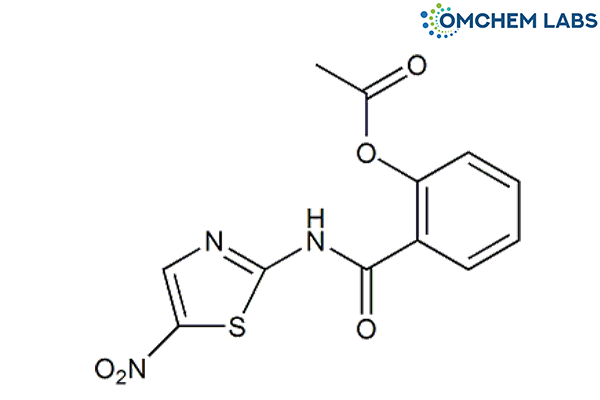
Nitazoxanide
| Catalogue No |
NITA-OCL-001 |
| CAS NO |
55981-09-4 |
| Molecular Formula | C12H9N3O5S |
| Molecular weight | 307.28 |
| Inquiry Status | In Stock |
| Synonyms | [2-[(5-Nitro-1,3-thiazol-2-yl)carbamoyl]phenyl]ethanoate |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Nitazoxanide: A Scientific Perspective
Introduction
The study and control of impurities associated with Nitazoxanide have become a critical area of focus in the pharmaceutical industry. As regulatory expectations intensify and the emphasis on drug safety and consistency increases, impurity profiling now plays a fundamental role in drug development and quality control. Any chemical entity, apart from the intended active pharmaceutical ingredient (API), that arises during manufacturing or storage must be identified, understood, and appropriately managed. In the case of Nitazoxanide, ensuring impurity levels remain within acceptable boundaries is essential to maintain therapeutic efficacy and patient safety, while also satisfying global regulatory requirements.
Formation of Impurities During API Synthesis
Impurities associated with Nitazoxanide can emerge from a wide variety of sources throughout the synthetic process. Common origins include incomplete reactions, side reactions, rearrangements, degradation pathways, and residuals from starting materials, reagents, or solvents. Process conditions such as temperature, pH, reaction time, and the nature of the synthetic route significantly influence the formation and diversity of these impurities. Additionally, post-synthesis factors like environmental exposure, humidity, light sensitivity, or oxidation can lead to transformation or degradation of the API, resulting in additional impurity profiles that require continuous monitoring and control.
Analytical Data Interpretation Techniques
Characterizing impurities in Nitazoxanide requires the implementation of robust and sensitive analytical methodologies. Instruments such as high-performance liquid chromatography (HPLC), gas chromatography (GC), mass spectrometry (MS), and nuclear magnetic resonance (NMR) are routinely utilized to separate, detect, and structurally elucidate impurities. These analytical platforms are essential not only for identifying known process-related substances but also for uncovering unknown or novel impurities. A thorough interpretation of chromatographic peaks, spectral signatures, and retention data allows researchers to differentiate impurities from the parent drug and to map impurity behavior under varying conditions.
Method Validation for Impurity Detection
Reliable impurity detection in Nitazoxanide depends on the validation of the analytical techniques employed. Validation is not only a regulatory necessity but also ensures consistency, accuracy, and sensitivity in detecting impurities at trace levels. The process typically includes the evaluation of parameters such as method specificity, reproducibility, linear response, detection limits, and robustness under varying experimental conditions. A properly validated method ensures that impurity data remains credible over time and across different manufacturing batches, providing a strong foundation for regulatory submissions and routine quality assessments.
Purification Strategies for Reducing Impurities
In order to deliver Nitazoxanide with optimal purity, targeted purification steps are integrated into the manufacturing process. The selection of an appropriate purification method is based on the physicochemical characteristics of both the API and its impurities. Techniques like crystallization, solvent extraction, column chromatography, and fractional distillation are employed depending on solubility, polarity, volatility, or molecular size differences. By refining these parameters, manufacturers can significantly reduce impurity levels, improve the overall quality profile, and enhance product stability.
Isolation and Characterization of Impurities
When impurity levels exceed established thresholds or if their structures are unknown, isolation becomes necessary for detailed examination. Techniques such as preparative HPLC, flash chromatography, and solid-phase extraction are utilized to separate impurities from Nitazoxanide in significant quantities for characterization. Structural elucidation is achieved through advanced spectroscopic techniques, including MS, NMR, and infrared spectroscopy. This process is crucial for confirming impurity identity, assessing potential toxicity, and preparing reference standards for future analysis and comparison.
Conclusion
The impurity profiling of Nitazoxanide represents a sophisticated integration of synthetic chemistry knowledge, analytical precision, regulatory compliance, and risk-based quality management. By understanding the origin of impurities, applying advanced analytical techniques, validating methods, and employing effective purification and isolation protocols, pharmaceutical developers can ensure the consistent production of high-quality Nitazoxanide. This approach not only supports regulatory approval but also upholds patient safety and therapeutic effectiveness, which are the core goals of pharmaceutical manufacturing.
