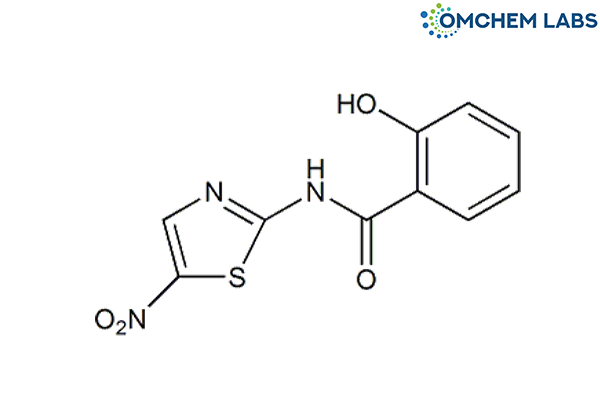
Tizoxanide || Nitazoxanide Desacetyl Impurity
| Catalogue No |
NITA-OCL-002 |
| CAS NO |
173903-47-4 |
| Molecular Formula | C10H7N3O4S |
| Molecular weight | 265.25 |
| Inquiry Status | In Stock |
| Synonyms | Tizoxanide 2-Hydroxy-N-(5-nitro-2-thiazolyl)benzamide |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Scientific Overview: Impurity Profiling of Nitazoxanide Desacetyl Impurity
Introduction
In pharmaceutical sciences, impurity profiling is a vital component of drug substance development and quality control. The identification and understanding of associated impurities—particularly transformation products like Nitazoxanide Desacetyl Impurity—are crucial for ensuring the safety and consistency of active pharmaceutical ingredients (APIs). These impurities may arise from synthetic procedures, degradation pathways, or formulation interactions. Profiling such entities is not only a regulatory necessity but also an integral part of risk mitigation throughout the drug development pipeline.
Formation of Impurities During API Synthesis
Impurities in an API like Nitazoxanide Desacetyl Impurity can originate from multiple stages in the synthetic process. These stages include incomplete reactions, over-processing, reagent remnants, and side reactions induced by specific conditions such as temperature, solvents, or pH variations. Additionally, transformation products may evolve during storage or formulation due to hydrolysis, oxidation, or photolysis. Structural modifications in such impurities often bear close resemblance to the parent compound, making their identification both critical and complex.
Analytical Data Interpretation Techniques
To monitor and understand the presence of Nitazoxanide Desacetyl Impurity, a broad range of analytical technologies is employed. Chromatographic tools such as high-performance liquid chromatography (HPLC) and ultra-performance liquid chromatography (UPLC), often paired with detectors like mass spectrometry (MS) or diode-array detection (DAD), serve as foundational techniques. For deeper structural insights, spectroscopic methods such as nuclear magnetic resonance (NMR) and infrared (IR) spectroscopy are applied. Interpretation of data from these instruments provides a qualitative and quantitative overview of impurity presence, aiding in source tracing and process refinement.
Method Validation for Impurity Detection
Any analytical method designed to detect or quantify Nitazoxanide Desacetyl Impurity must undergo thorough validation before routine use. Validation ensures that the method is reliable, reproducible, and sensitive enough to capture trace-level impurities. Key parameters considered in method validation include specificity, linearity, detection thresholds, accuracy, and robustness. By adhering to international standards and regulatory frameworks, validated methods contribute to the overall integrity of impurity assessments and batch release decisions.
Purification Strategies for Reducing Impurities
Reducing the level of impurities like Nitazoxanide Desacetyl Impurity requires tailored purification strategies that depend on the impurity’s chemical and physical properties. Commonly used approaches include recrystallization, solvent switching, liquid-liquid extraction, and preparative chromatography. Each method aims to either remove or minimize undesired components while retaining the efficacy and yield of the API. Optimization of these strategies is often iterative, evolving as new impurities are identified or manufacturing conditions are scaled up.
Isolation and Characterization of Impurities
When an impurity crosses predefined regulatory thresholds or remains unidentified, it becomes necessary to isolate and characterize it fully. For Nitazoxanide Desacetyl Impurity, isolation is typically achieved through preparative-scale chromatographic techniques. Post-isolation, structural elucidation involves detailed spectroscopic analysis using NMR, MS, and IR technologies. Understanding the molecular structure not only assists in toxicological risk assessment but also enables the establishment of impurity-specific reference standards for future analytical comparisons.
Conclusion
The comprehensive profiling of Nitazoxanide Desacetyl Impurity illustrates the complexity and necessity of controlling chemical variability in pharmaceutical products. From origin and detection to purification and structural analysis, each phase contributes to the overarching goal of ensuring drug safety, consistency, and compliance. A systematic and science-driven approach to impurity management not only satisfies regulatory expectations but also supports the pharmaceutical industry's broader mission of delivering high-quality therapeutics to patients.
