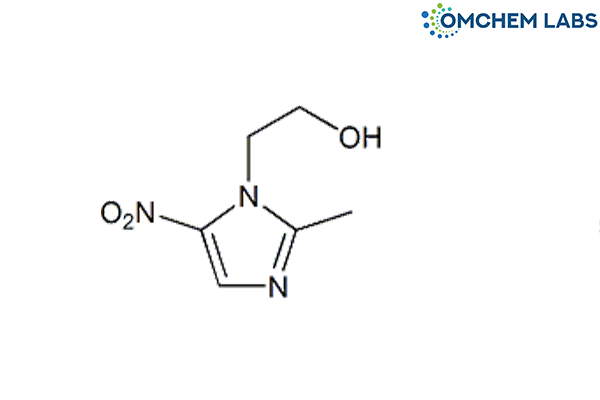
Metronidazole
| Catalogue No |
METR-OCL-001 |
| CAS NO |
443-48-1 |
| Molecular Formula | C6H9N3O3 |
| Molecular weight | 171.15 |
| Inquiry Status | In Stock |
| Synonyms | 1-(2-Hydroxyethyl)-2-methyl-5-nitroimidazole |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Metronidazole: A Scientific Overview
Introduction
The pharmaceutical quality and therapeutic reliability of an active pharmaceutical ingredient (API) such as Metronidazole are fundamentally linked to its impurity profile. Impurity profiling encompasses the identification, structural elucidation, and control of unwanted chemical entities that may arise at any stage of the drug development process. These impurities, though often present in trace amounts, can influence pharmacological outcomes and regulatory acceptance. With increasing global scrutiny from health authorities, establishing a comprehensive impurity management approach for Metronidazole is not just a regulatory requirement, but also a scientific imperative to ensure patient safety and drug efficacy.
Formation of Impurities During API Synthesis
Impurities in Metronidazole may be introduced through multiple sources during its synthesis, including raw material residues, intermediates, degradation products, and unintended reaction by-products. Often, reaction conditions—such as temperature, pressure, pH, and catalyst type—play a major role in the development of side reactions that give rise to chemical contaminants. Moreover, post-synthesis processes like drying, filtration, or storage under non-ideal conditions can also contribute to the formation of new impurities or the transformation of existing ones. A proactive understanding of the synthetic pathway enables early prediction and minimization of such impurities during process development.
Analytical Data Interpretation Techniques
Thorough impurity profiling of Metronidazole relies on the deployment of high-resolution analytical technologies. Techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and liquid chromatography coupled with mass spectrometry (LC-MS) are widely adopted to detect, separate, and identify trace-level impurities. Nuclear magnetic resonance (NMR) spectroscopy and Fourier-transform infrared spectroscopy (FTIR) also assist in structural characterization. The accurate interpretation of spectral and chromatographic data enables clear differentiation between the API and structurally similar impurities. Such interpretation demands a deep understanding of molecular behavior, peak patterns, and retention profiles in diverse chromatographic conditions.
Method Validation for Impurity Detection
Analytical methods used to examine impurities in Metronidazole must undergo rigorous validation to confirm their reliability and reproducibility. Following international standards and regulatory guidance, validation typically involves evaluating parameters like specificity, accuracy, precision, detection limit, quantitation threshold, linearity, and robustness. These attributes ensure that the chosen method can consistently detect minor components even in complex mixtures. Method validation not only enhances the credibility of the impurity profiling process but also supports the generation of defensible data for regulatory submissions.
Purification Strategies for Reducing Impurities
Once identified, impurities in Metronidazole must be minimized through efficient purification protocols. The selection of a suitable purification technique depends on the physical and chemical characteristics of both the API and the impurities. Crystallization, solvent extraction, distillation, and preparative chromatography are among the commonly utilized approaches. Crystallization, for instance, can selectively exclude impurities based on solubility differences, while chromatography offers high-resolution separation based on molecular interactions. The optimization of these strategies ensures high purity without compromising yield or process efficiency.
Isolation and Characterization of Impurities
In cases where impurities in Metronidazole exceed regulatory thresholds or are previously unreported, it becomes essential to isolate and characterize them. Isolation is typically conducted using preparative-scale chromatographic techniques tailored to the impurity’s properties. After isolation, structural elucidation is achieved through advanced spectroscopic methods like NMR, MS, and IR. These tools reveal critical information about the impurity’s functional groups, molecular framework, and stereochemistry. Isolated and characterized impurities may also be developed into reference standards for routine use in quality control laboratories, supporting long-term consistency.
Conclusion
Impurity profiling of Metronidazole is a scientifically intricate and regulatory-driven process that spans synthesis monitoring, analytical evaluation, method validation, purification, and structural elucidation. Each stage in the impurity management lifecycle contributes to ensuring that the API meets quality benchmarks for safety and therapeutic performance. A well-structured impurity control strategy not only reinforces product integrity but also aligns with international expectations for pharmaceutical excellence. By embracing robust scientific principles and modern analytical technologies, the impurity profile of Metronidazole can be effectively understood, controlled, and documented across all phases of development and manufacturing.
