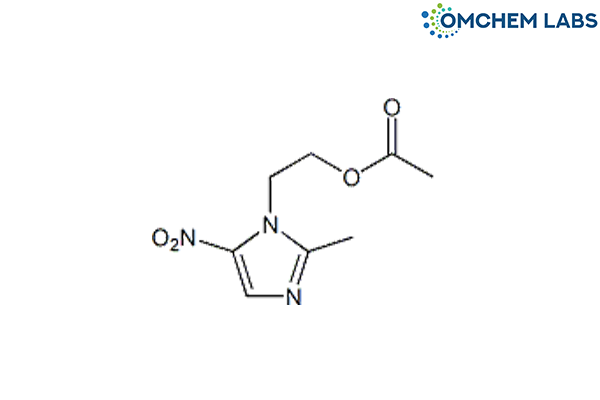
Metronidazole Acetate
| Catalogue No |
METR-OCL-002 |
| CAS NO |
13182-82-6 |
| Molecular Formula | C8H11N3O4 |
| Molecular weight | 213.19 |
| Inquiry Status | In Stock |
| Synonyms | 2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethyl acetate |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Metronidazole Acetate: A Scientific Perspective
Introduction
The quality of pharmaceutical substances is critically dependent on their chemical purity, particularly with respect to impurities that may arise during synthesis, storage, or handling. Impurity profiling of Metronidazole Acetate is a systematic scientific process that enables identification, evaluation, and control of undesired chemical entities associated with the active pharmaceutical ingredient (API). Regulatory authorities emphasize the importance of impurity profiling due to potential implications on product safety, stability, and therapeutic efficacy. Establishing a thorough understanding of the impurity landscape ensures consistency in manufacturing and compliance with international quality guidelines.
Formation of Impurities During API Synthesis
Impurities in Metronidazole Acetate may originate from multiple sources throughout the production lifecycle. These include reaction by-products, unreacted precursors, intermediate compounds, residual solvents, and degradants formed due to environmental conditions such as temperature, humidity, or light exposure. Reaction mechanisms, catalysts employed, pH levels, and reaction durations all play pivotal roles in determining the nature and quantity of potential impurities. Additionally, impurities may evolve during downstream processes such as crystallization or drying, making a comprehensive synthesis control strategy vital for impurity minimization.
Analytical Data Interpretation Techniques
Accurate impurity detection and identification in Metronidazole Acetate is enabled by sophisticated analytical tools that offer high sensitivity and specificity. Techniques like high-performance liquid chromatography (HPLC), gas chromatography (GC), liquid chromatography coupled with mass spectrometry (LC-MS), and nuclear magnetic resonance (NMR) spectroscopy are extensively applied. These instruments allow analysts to differentiate between the parent compound and associated impurities based on retention time, mass spectra, or structural features. Analytical interpretation requires in-depth understanding of spectral patterns, chromatographic resolution, and baseline stability to confidently classify both known and novel impurities.
Method Validation for Impurity Detection
Before impurity data can be considered reliable, the analytical procedures used for their determination must be validated under controlled conditions. For Metronidazole Acetate, method validation ensures that the detection process is consistent, reproducible, and accurate across various conditions. Parameters such as specificity, precision, linearity, and detection thresholds are examined to demonstrate the robustness of the analytical system. Adhering to standardized validation protocols is essential not only for internal quality assurance but also for compliance with regulatory documentation requirements.
Purification Strategies for Reducing Impurities
Once impurities are identified and characterized, suitable purification processes are employed to reduce them to acceptable levels. For Metronidazole Acetate, the selection of purification techniques is largely guided by the physicochemical properties of both the target compound and its impurities. Methods such as recrystallization, solvent extraction, distillation, and column chromatography are often considered. An optimized purification strategy aims to maximize the removal of impurities while maintaining high yield and integrity of the API. The purification process is typically refined through experimental iteration and risk assessment to ensure optimal impurity control.
Isolation and Characterization of Impurities
In scenarios where certain impurities in Metronidazole Acetate exceed regulatory thresholds or are uncharacterized, isolation becomes necessary for further study. Advanced separation techniques, such as preparative HPLC or flash chromatography, are employed to isolate impurities in sufficient quantity and purity. Following isolation, spectroscopic methods including NMR, MS, and FTIR are used for structural elucidation. Characterization of impurity structure supports toxicological risk assessments and facilitates development of impurity reference standards, both of which are crucial for regulatory submission and long-term quality monitoring.
Conclusion
Impurity profiling of Metronidazole Acetate is a multidimensional process that integrates chemical synthesis understanding, advanced analytical evaluation, method validation, strategic purification, and rigorous structural analysis. This approach ensures that all impurities are systematically identified, controlled, and monitored throughout the API lifecycle. By adopting a science-based, proactive strategy for impurity management, pharmaceutical developers can ensure the safety, efficacy, and regulatory compliance of their products. A comprehensive impurity profiling framework not only reinforces product quality but also supports sustainable pharmaceutical development.
