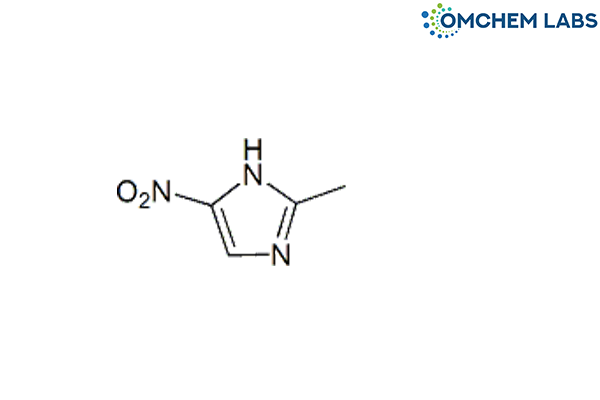
Metronidazole Impurity A
| Catalogue No |
METR-OCL-003 |
| CAS NO |
696-23-1 |
| Molecular Formula | C4H5N3O2 |
| Molecular weight | 127.10 |
| Inquiry Status | In Stock |
| Synonyms | 2-Methyl-4-nitroimidazole |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Metronidazole Impurity A: Scientific Insights into Assessment and Control
Introduction
The presence and behavior of impurities in active pharmaceutical ingredients (APIs) have become critical areas of scrutiny in pharmaceutical research and regulatory science. Metronidazole Impurity A represents one such structurally or process-related impurity that must be accurately profiled to ensure the final drug substance maintains therapeutic efficacy and patient safety. The identification, control, and characterization of impurities are not just regulatory mandates, but integral components of quality-by-design approaches in drug manufacturing. In the context of Metronidazole synthesis, impurity profiling provides insights into the chemical robustness of the production process and offers early indicators for potential product degradation or formulation challenges.
Formation of Impurities During API Synthesis
The synthesis of Metronidazole, like many APIs, involves multiple synthetic intermediates, reaction conditions, and purification steps. Impurity A may originate at various stages—whether as a side-product of the primary reaction, an unreacted precursor, a degradation product, or even a transformation under post-process storage. Factors such as solvent choice, catalyst type, reaction temperature, and duration all play a role in impurity generation. Even small changes in synthetic parameters can result in altered impurity patterns. Understanding the kinetic and thermodynamic conditions that lead to the formation of Impurity A allows for upstream control and smarter design of synthetic routes.
Analytical Data Interpretation Techniques
Profiling impurities demands not only detection but clear interpretation of analytical results. To assess Metronidazole Impurity A, a suite of analytical platforms is generally applied. These include chromatographic techniques for separation and quantification and spectroscopic tools for structural elucidation. Chromatographic retention times, UV absorption patterns, and mass fragmentation data offer a comprehensive view of the impurity landscape. When used synergistically, these data sources provide a robust interpretative framework that enables the clear identification and monitoring of even trace-level impurities. Interpreting this data accurately is essential for setting justified limits and identifying shifts in the synthetic process or material quality.
Method Validation for Impurity Detection
Analytical methods used for impurity profiling are only as reliable as the validation process behind them. For the quantification and detection of Metronidazole Impurity A, methods must be systematically validated to demonstrate their suitability. This includes evaluating attributes such as sensitivity, specificity, precision, and repeatability. The goal is to confirm that the method consistently identifies the impurity without interference from other components or excipients. Regulatory guidance recommends that validation be an ongoing, lifecycle-based activity, evolving as process knowledge and analytical capabilities advance. A well-validated method ensures that impurity control is not a reactive task but a built-in quality system.
Purification Strategies for Reducing Impurities
Once impurities are identified and characterized, mitigation becomes the next logical step. Reducing Metronidazole Impurity A often involves refining purification protocols, adjusting crystallization parameters, or implementing selective extraction techniques. The choice of purification strategy hinges on the impurity’s physicochemical properties—solubility, polarity, volatility—and its interaction with the API and process solvents. Effective purification is not just about eliminating unwanted compounds but doing so without compromising the integrity or yield of the target molecule. Tailoring the purification process to specifically remove Impurity A, without triggering new impurity pathways, reflects a well-engineered process strategy.
Isolation and Characterization of Impurities
When the impurity cannot be confidently identified through in-situ analysis alone, isolation becomes necessary. This is particularly relevant when Metronidazole Impurity A reaches significant levels or exhibits unexpected behavior during stability studies. Isolation typically involves preparative chromatographic techniques or crystallization, followed by structural characterization using methods such as NMR spectroscopy, mass spectrometry, and IR analysis. These tools enable chemists to construct a molecular-level understanding of the impurity, including its functional groups and potential routes of formation. Once characterized, Impurity A can be benchmarked against safety thresholds and toxicological profiles, supporting decisions about specification setting and regulatory acceptability.
Conclusion
Profiling of Metronidazole Impurity A is a multidimensional effort involving synthetic chemistry, advanced analytics, method validation, and strategic purification. Each step contributes to a holistic understanding of impurity behavior within the drug substance matrix. As pharmaceutical quality standards continue to evolve, the role of impurity science grows increasingly central to product development and lifecycle management. By proactively addressing the identification, quantification, and control of Metronidazole Impurity A, manufacturers can ensure not only regulatory compliance but also the long-term quality and safety of the therapeutic product.
