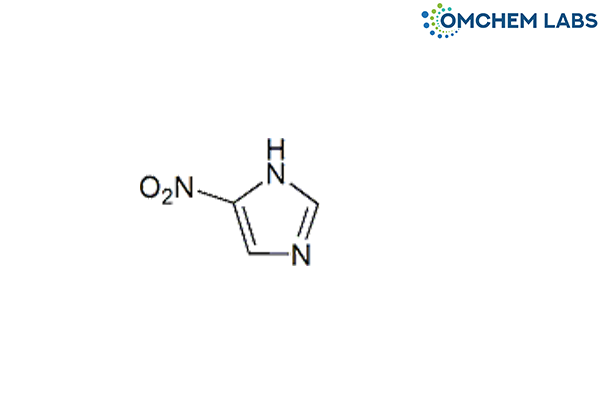
Metronidazole Impurity B
| Catalogue No |
METR-OCL-004 |
| CAS NO |
3034-38-6 |
| Molecular Formula | C3H3N3O2 |
| Molecular weight | 113.07 |
| Inquiry Status | In Stock |
| Synonyms | 4(5)-Nitroimidazole |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Metronidazole Impurity B: Scientific Overview and Strategic Approaches
Introduction
Ensuring the chemical integrity of pharmaceutical substances requires a thorough understanding of impurities, especially those that may emerge throughout the manufacturing life cycle. Metronidazole Impurity B represents one such impurity, whose identification and control are essential for maintaining the quality and safety of the final drug product. As part of regulatory expectations and good manufacturing practices, impurity profiling provides insight into the synthetic pathways, environmental sensitivities, and potential degradation of the active pharmaceutical ingredient (API). Effective profiling not only enhances product quality but also supports global compliance and patient safety.
Formation of Impurities During API Synthesis
Impurities such as Metronidazole Impurity B typically arise from a variety of sources during the synthesis of the active compound. These may include leftover starting materials, incomplete reactions, formation of by-products due to side reactions, or degradation that occurs under processing or storage conditions. The complexity of multistep synthesis can also introduce intermediate compounds that persist into the final stage. Environmental factors such as heat, moisture, pH variations, and light exposure further contribute to impurity generation. A clear understanding of synthetic routes and process variables is key to identifying potential impurity risks early in development.
Analytical Data Interpretation Techniques
Accurate impurity profiling hinges on the use of advanced analytical instrumentation capable of detecting trace-level components in complex matrices. Techniques like high-performance liquid chromatography (HPLC), gas chromatography (GC), and hyphenated methods such as LC-MS and GC-MS play a pivotal role in tracking and differentiating impurities from the API. Spectroscopic tools, including nuclear magnetic resonance (NMR) and infrared (IR) spectroscopy, aid in structural elucidation. Data interpretation focuses not only on identifying impurity peaks but also on understanding their chemical relationships and origin within the process. Careful analytical review helps avoid false positives and supports more targeted control strategies.
Method Validation for Impurity Detection
Before relying on analytical procedures for routine impurity analysis, validation ensures their scientific robustness and regulatory acceptability. When profiling Metronidazole Impurity B, analytical methods must demonstrate reliability across a range of critical parameters such as precision, specificity, linearity, and sensitivity. The ability of the method to detect and quantify minor levels of the impurity without interference from the matrix is crucial. Method validation provides assurance that impurity measurements are consistent, repeatable, and meaningful, forming the foundation of quality control throughout the API's life cycle.
Purification Strategies for Reducing Impurities
Reducing or eliminating impurities involves strategic purification methods tailored to the physicochemical properties of the compound and its associated contaminants. For Metronidazole Impurity B, options may include crystallization, liquid-liquid extraction, adsorption techniques, and preparative chromatographic separations. The goal is to optimize purification efficiency while minimizing loss of the active component. Choice of solvents, temperature controls, and process timing all influence the success of impurity removal. Proper purification not only enhances product purity but also simplifies downstream analytical monitoring.
Isolation and Characterization of Impurities
When an impurity reaches significant levels or remains structurally unknown, isolation becomes necessary for in-depth study. The separation of Metronidazole Impurity B from the main compound can be achieved using scaled-up chromatographic techniques, followed by characterization through NMR, MS, and other spectroscopic analyses. Understanding the impurity’s structure and origin allows developers to modify the synthesis route or introduce control steps. Additionally, isolated impurities may be used to develop reference standards for future analytical use, supporting more robust process validation and regulatory submission.
Conclusion
The profiling of Metronidazole Impurity B reflects a broader scientific effort to ensure pharmaceutical purity, safety, and regulatory compliance. From understanding impurity formation mechanisms to refining analytical methods, each phase of the impurity lifecycle contributes to a more reliable and consistent drug development process. Through comprehensive validation, efficient purification, and detailed structural analysis, impurity profiling becomes not just a regulatory obligation but a scientific necessity for delivering high-quality medicines. An integrated approach to impurity control is essential for upholding the integrity of the pharmaceutical supply chain and protecting patient health.
