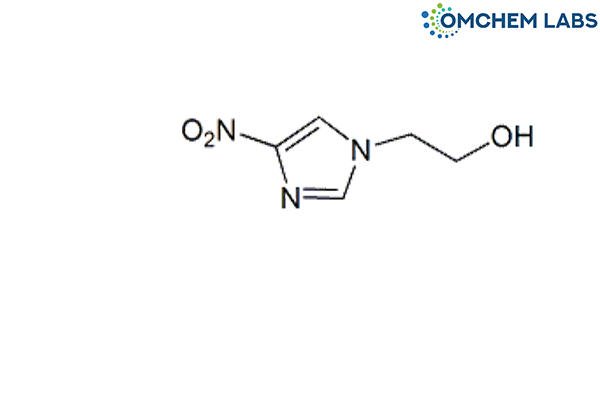
Metronidazole Impurity C
| Catalogue No |
METR-OCL-005 |
| CAS NO |
5006-69-9 |
| Molecular Formula | C5H7N3O3 |
| Molecular weight | 157.13 |
| Inquiry Status | In Stock |
| Synonyms | 2-(4-Nitro-1H-imidazol-1-yl)ethanol ; 4-Nitro-1H-imidazole-1-ethanol |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Metronidazole Impurity C: A Strategic Scientific Approach
Introduction
Pharmaceutical quality assurance relies heavily on comprehensive impurity profiling, particularly when it involves structurally related or process-derived by-products such as Metronidazole Impurity C. The presence of impurities, even in trace amounts, can influence the pharmacokinetics, safety, and efficacy of a drug substance. Regulatory authorities emphasize the necessity of identifying and controlling impurities to ensure consistent therapeutic performance and patient safety. As such, impurity profiling is not merely a compliance requirement but an integral component of pharmaceutical development and lifecycle management.
Formation of Impurities During API Synthesis
The synthesis of active pharmaceutical ingredients like Metronidazole can give rise to related impurities due to several intrinsic and extrinsic factors. Metronidazole Impurity C may emerge as a result of incomplete reactions, over-reaction, rearrangement, degradation of intermediates, or interaction with residual reagents and solvents. The reaction environment—including parameters such as temperature, pH, time, and solvent polarity—also plays a crucial role in the formation and variability of impurity profiles. Additionally, process changes, scale-up modifications, and storage conditions can introduce new or unexpected impurity species, making routine assessment vital.
Analytical Data Interpretation Techniques
To accurately monitor and profile Metronidazole Impurity C, sophisticated analytical methodologies are employed. Techniques like high-performance liquid chromatography (HPLC), gas chromatography (GC), mass spectrometry (MS), and nuclear magnetic resonance (NMR) spectroscopy provide the resolution and sensitivity needed to detect impurities at low thresholds. The interpretation of chromatograms, spectral signatures, and molecular fragmentation patterns enables the differentiation of target compounds from unwanted species. This analytical insight lays the groundwork for setting specifications and controlling impurities throughout production and stability studies.
Method Validation for Impurity Detection
Reliable impurity detection hinges on the robustness of the analytical techniques used. In the context of Metronidazole Impurity C, analytical method validation ensures that the chosen procedures deliver consistent, accurate, and reproducible results across batches. Parameters such as specificity, accuracy, precision, detection limits, and robustness are critically assessed in line with global regulatory standards. The goal is to confirm that the method can effectively distinguish the impurity from the main compound and other possible interfering substances under varied analytical conditions.
Purification Strategies for Reducing Impurities
Once impurities are identified and quantified, refining the purification process becomes essential. A range of techniques—such as selective crystallization, liquid-liquid extraction, recrystallization, and preparative chromatography—can be employed to minimize the presence of Metronidazole Impurity C. These techniques are selected based on the chemical nature of the impurity and its behavior relative to the desired API. Optimizing purification not only enhances the purity profile but also improves process efficiency, regulatory compliance, and long-term product stability.
Isolation and Characterization of Impurities
For impurities that persist beyond acceptable thresholds or lack structural clarity, isolation and characterization are necessary. Isolation typically involves scaling up chromatographic techniques to separate sufficient quantities of the impurity for study. Structural elucidation of Metronidazole Impurity C is carried out using a combination of spectral tools such as MS, NMR, and IR spectroscopy. These methods help establish the molecular framework, which is crucial for toxicological evaluation and defining impurity limits in the finished product. Developing a reference standard may also be warranted for recurring impurities to facilitate routine monitoring.
Conclusion
The impurity profiling of Metronidazole Impurity C illustrates the critical intersection of synthetic chemistry, analytical science, and regulatory strategy in pharmaceutical development. From formation pathways to analytical detection, validation, purification, and structural investigation, each step contributes to building a comprehensive quality profile for the API. A robust and scientifically sound impurity management approach not only ensures regulatory adherence but also underpins the therapeutic integrity and patient safety of the pharmaceutical product.
