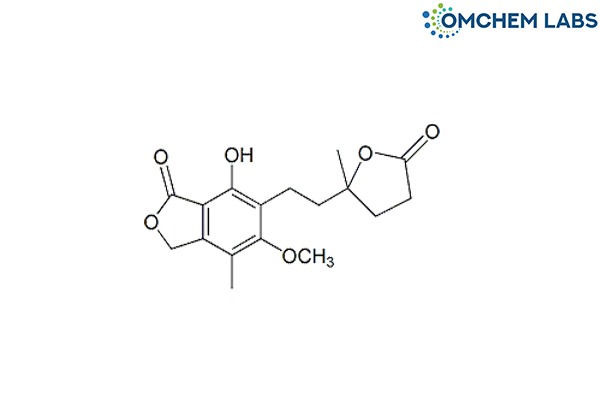
Mycophenolate Mofetil Related Compound B
| Catalogue No |
MYCO-OCL-008 |
| CAS NO |
26675-76-3 |
| Molecular Formula | C17H20O6 |
| Molecular weight | 320.34 |
| Inquiry Status | In Stock |
| Synonyms | (RS)-7-Hydroxy-5-methoxy-4-methyl-6-[2-(5-methyl-2-oxo-tetrahydro-furan-5-yl)-ethyl]-3H-isobenzofuranyl-1-one |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Mycophenolate Related Compound B: A Comprehensive Overview
Introduction
Impurity profiling of Mycophenolate Related Compound B is an essential aspect of pharmaceutical development and quality assurance. Impurities, whether process-related, degradation-based, or introduced through environmental exposure, can significantly impact the safety, efficacy, and regulatory acceptance of the final active pharmaceutical ingredient (API). Understanding and controlling these impurities is vital to ensure the consistent quality of pharmaceutical products and compliance with global standards.
Formation of Impurities During API Synthesis
During the synthesis of Mycophenolate Related Compound B, impurities may arise at various stages, including raw material handling, chemical reactions, and purification steps. These can include unreacted starting materials, intermediates, degradation products, side reaction by-products, residual solvents, and reagents. Factors such as reaction conditions, temperature, pH, and the presence of catalysts also influence impurity formation. Moreover, storage conditions after synthesis may lead to additional degradation impurities, making it crucial to evaluate both process-related and post-process impurity profiles.
Analytical Data Interpretation Techniques
Advanced analytical techniques are employed to detect, identify, and quantify impurities in Mycophenolate Related Compound B. High-performance liquid chromatography (HPLC), gas chromatography (GC), liquid chromatography–mass spectrometry (LC-MS), and nuclear magnetic resonance (NMR) spectroscopy are among the most widely used methods. These tools allow for precise determination of impurity structures, retention times, mass-to-charge ratios, and spectral patterns. Chromatographic and spectroscopic data are interpreted to differentiate between known and unknown impurities, ensuring batch-to-batch consistency and compliance with pharmacopeial specifications.
Method Validation for Impurity Detection
To ensure the reliability of analytical methods used in impurity profiling, rigorous validation is performed following international guidelines such as ICH Q2(R1). Critical validation parameters include accuracy, precision, specificity, linearity, limit of detection (LOD), limit of quantitation (LOQ), robustness, and range. Validated methods are essential for demonstrating that impurities in Mycophenolate Related Compound B can be consistently detected and quantified at trace levels. This step also supports regulatory submissions by proving the credibility and reproducibility of the analytical results.
Purification Strategies for Reducing Impurities
Purification is a key step in controlling the impurity levels in Mycophenolate Related Compound B. Techniques such as crystallization, solvent extraction, distillation, and preparative chromatography are commonly employed. Crystallization is particularly effective when impurities have significantly different solubility profiles compared to the target compound. Distillation is suitable for removing volatile contaminants, while chromatographic methods offer higher resolution for separating structurally similar impurities. An optimized purification strategy ensures high product purity and enhances the overall yield.
Isolation and Characterization of Impurities
When an impurity in Mycophenolate Related Compound B exceeds identification thresholds or poses a potential risk, it must be isolated and fully characterized. Isolation is typically achieved using preparative HPLC or flash chromatography. Subsequent characterization involves spectroscopic techniques such as NMR, MS, and IR spectroscopy to elucidate the impurity's chemical structure. Proper identification is critical for toxicological evaluation, regulatory documentation, and setting acceptable limits. In some cases, reference standards are developed for recurrent impurities to support ongoing analytical testing.
Conclusion
The impurity profiling of Mycophenolate Related Compound B is a comprehensive and multidimensional process involving synthesis understanding, advanced analytical techniques, rigorous validation, effective purification strategies, and precise impurity characterization. Each step plays a vital role in ensuring the safety, efficacy, and quality of pharmaceutical products. A robust impurity control strategy is not only a regulatory requirement but also a critical component of patient safety and product lifecycle management.
