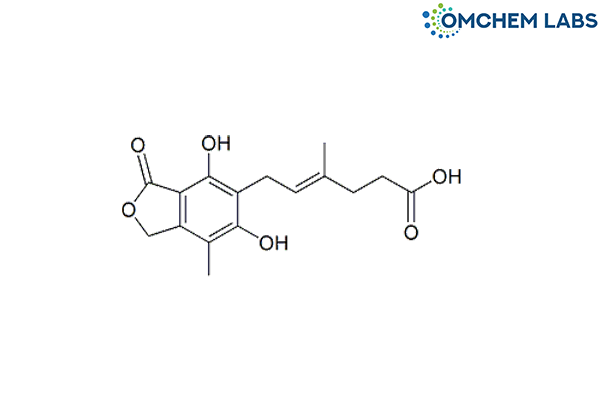
Mycophenolic Acid O-Desmethyl Impurity
| Catalogue No |
MYCO-OCL-011 |
| CAS NO |
31858-65-8 |
| Molecular Formula | C16H18O6 |
| Molecular weight | 306.31 |
| Inquiry Status | In Stock |
| Synonyms | (4E)-6-(1,3-Dihydro-4,6-dihydroxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoic acid |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Mycophenolic Acid O-Desmethyl Impurity: A Scientific Perspective
Introduction
The comprehensive understanding and regulation of pharmaceutical impurities play a vital role in ensuring the safety, efficacy, and regulatory compliance of active pharmaceutical ingredients (APIs). Among these, Mycophenolic Acid O-Desmethyl Impurity presents a specific interest due to its structural relationship with the parent compound and its potential presence as a process or degradation-related impurity. Impurity profiling serves as a systematic framework to investigate such associated components, which may arise during manufacturing or storage. This scientific process supports the pharmaceutical industry's efforts to deliver consistent, high-quality medicines while meeting global regulatory expectations.
Formation of Impurities During API Synthesis
The generation of impurities such as Mycophenolic Acid O-Desmethyl Impurity can occur through a range of pathways throughout the synthetic route of an API. These pathways may include incomplete reactions, undesired side reactions, or the presence of reactive intermediates. Reagent or catalyst residues, solvent traces, and changes in processing conditions like temperature or pH also contribute to impurity formation. In addition, post-synthesis handling, such as drying or packaging, can induce chemical transformations leading to the formation or increase of related impurities. Understanding these origins is critical in developing a robust control strategy throughout the drug development lifecycle.
Analytical Data Interpretation Techniques
Accurate impurity profiling hinges on the application and interpretation of advanced analytical techniques. For impurities like Mycophenolic Acid O-Desmethyl Impurity, chromatographic and spectroscopic methods are often integrated to obtain a complete chemical fingerprint. Tools such as high-performance liquid chromatography (HPLC), mass spectrometry (MS), and nuclear magnetic resonance (NMR) enable detailed insight into chemical composition, molecular structure, and impurity behavior. Analytical interpretation not only confirms the identity of the impurity but also supports its distinction from structurally similar compounds. The use of orthogonal methods helps ensure comprehensive profiling with improved reliability.
Method Validation for Impurity Detection
Method validation is an essential phase that assures the performance of analytical procedures designed to detect impurities. In the case of Mycophenolic Acid O-Desmethyl Impurity, validation parameters such as precision, specificity, linearity, accuracy, and sensitivity must be considered in accordance with global regulatory guidelines. A validated method provides confidence in reproducibility and ensures the method can consistently detect the impurity at trace levels. This reliability is particularly crucial in meeting regulatory expectations and in demonstrating the robustness of both the method and the manufacturing process from which the impurity may arise.
Purification Strategies for Reducing Impurities
Once impurities have been identified and quantified, effective purification strategies must be implemented to reduce or eliminate them. For Mycophenolic Acid O-Desmethyl Impurity, the approach may involve physical and chemical separation techniques tailored to exploit differences in solubility, volatility, polarity, or molecular weight. Techniques such as selective crystallization, solvent extraction, or preparative chromatography can be applied depending on the nature of the impurity and its relation to the API. Developing a sound purification strategy is critical not only for ensuring product purity but also for improving yield and overall process efficiency.
Isolation and Characterization of Impurities
When an impurity exceeds specified thresholds or remains structurally undefined, isolation becomes a necessary step for further characterization. Mycophenolic Acid O-Desmethyl Impurity may be isolated through advanced preparative techniques like column chromatography or solid-phase extraction. Once isolated, the impurity undergoes structural elucidation using methods such as NMR, IR, and MS to determine its configuration, functional groups, and potential reactivity. Characterization is crucial for toxicological evaluation, regulatory submissions, and the creation of reference standards for future analytical work.
Conclusion
The impurity profiling of Mycophenolic Acid O-Desmethyl Impurity represents a multifaceted process requiring interdisciplinary expertise in synthetic chemistry, analytical science, and regulatory compliance. From understanding the routes of impurity formation to deploying precise analytical tools, validating methods, and applying appropriate purification and isolation techniques—each step contributes to a comprehensive impurity control strategy. Such profiling is integral not only to safeguarding public health but also to supporting the development and approval of high-quality pharmaceutical products. Establishing detailed and reliable impurity knowledge remains an ongoing commitment in pharmaceutical science and quality assurance.
