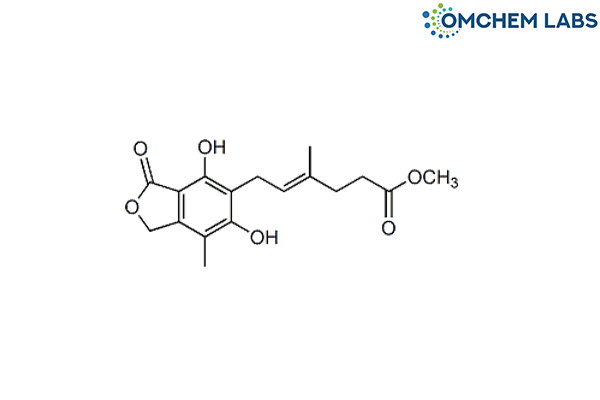
Mycophenolic Acid O-Desmethyl Methyl Ester
| Catalogue No |
MYCO-OCL-012 |
| CAS NO |
33431-38-8 |
| Molecular Formula | C17H20O6 |
| Molecular weight | 320.34 |
| Inquiry Status | In Stock |
| Synonyms | Methyl (4E)-6-(1,3-dihydro-4,6-dihydroxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Mycophenolic Acid O-Desmethyl Methyl Ester: A Scientific Perspective
Introduction
The comprehensive assessment of impurities is an integral part of pharmaceutical development, particularly for structurally related compounds such as Mycophenolic Acid O-Desmethyl Methyl Ester. Ensuring chemical purity in an active pharmaceutical ingredient (API) is not merely a regulatory requirement but a cornerstone of patient safety and therapeutic consistency. In the context of modern pharmaceutical manufacturing, impurity profiling involves a synergistic approach that combines synthetic chemistry, analytical science, and regulatory strategy. Understanding the origin, behavior, and control of impurities helps mitigate quality risks while improving the robustness of production processes.
Formation of Impurities During API Synthesis
Impurities associated with Mycophenolic Acid O-Desmethyl Methyl Ester may emerge from a variety of sources throughout the manufacturing process. They can be categorized broadly as process-related or degradation-related. Process impurities typically originate from unreacted starting materials, reaction by-products, or transformation products arising from intermediate steps. Variables such as reagent quality, solvent selection, catalyst use, and reaction conditions significantly influence impurity formation. Additionally, degradation impurities may form post-synthesis due to environmental stressors like moisture, heat, or pH shifts. These pathways underscore the need for a robust synthetic design and environmental control strategy.
Analytical Data Interpretation Techniques
Accurate impurity profiling hinges on the effective application of advanced analytical methodologies. For Mycophenolic Acid O-Desmethyl Methyl Ester, analytical evaluation often leverages high-resolution and orthogonal techniques such as chromatographic separation, mass spectrometry, and spectroscopic analysis. Chromatographic methods like HPLC or UHPLC are typically used to isolate impurity peaks, while MS and NMR support structure elucidation and identification. Interpreting the data generated by these platforms requires a nuanced understanding of peak purity, retention behavior, spectral fingerprinting, and fragmentation patterns. This analytical triangulation ensures a comprehensive impurity profile even in the presence of complex matrices.
Method Validation for Impurity Detection
Reliable impurity detection demands rigorously validated analytical procedures. The validation process ensures that methods used to quantify impurities in Mycophenolic Acid O-Desmethyl Methyl Ester meet established standards for sensitivity, selectivity, and reproducibility. Parameters such as specificity, linearity, limit of detection (LOD), and limit of quantification (LOQ) are assessed systematically to confirm performance. Additionally, method robustness is evaluated under varied conditions to simulate potential manufacturing variations. These efforts collectively reinforce the reliability of impurity testing and provide the confidence needed for regulatory compliance and long-term quality assurance.
Purification Strategies for Reducing Impurities
Minimizing impurity levels in the final product involves carefully selected purification strategies that are both scientifically sound and operationally feasible. For Mycophenolic Acid O-Desmethyl Methyl Ester, options such as recrystallization, solvent extraction, preparative chromatography, and phase separation may be utilized depending on the impurity profile. The choice of purification technique is influenced by the chemical nature of both the API and its impurities—such as solubility, polarity, and thermal stability. Implementing a purification plan that targets specific impurity classes improves the overall purity while supporting cost-effective manufacturing.
Isolation and Characterization of Impurities
When an impurity exceeds acceptable thresholds or lacks prior identification, its isolation becomes necessary. The process typically involves enrichment using chromatographic methods followed by structural elucidation through techniques such as NMR spectroscopy, infrared analysis, and mass spectrometry. Isolated impurities in Mycophenolic Acid O-Desmethyl Methyl Ester are then studied to understand their formation pathways, potential toxicological impact, and stability profile. Establishing reference standards for recurrent impurities also facilitates routine analysis and supports consistency across multiple production lots.
Conclusion
Impurity profiling of Mycophenolic Acid O-Desmethyl Methyl Ester is a multi-dimensional task that integrates synthesis, analysis, validation, and purification into a unified quality control framework. A well-designed impurity control strategy not only aligns with regulatory expectations but also ensures the therapeutic consistency and safety of the pharmaceutical product. The development of impurity knowledge early in the product lifecycle enables proactive risk management, continuous process improvement, and long-term product integrity. As pharmaceutical sciences evolve, the precision and depth of impurity profiling remain central to the credibility and success of pharmaceutical development.
