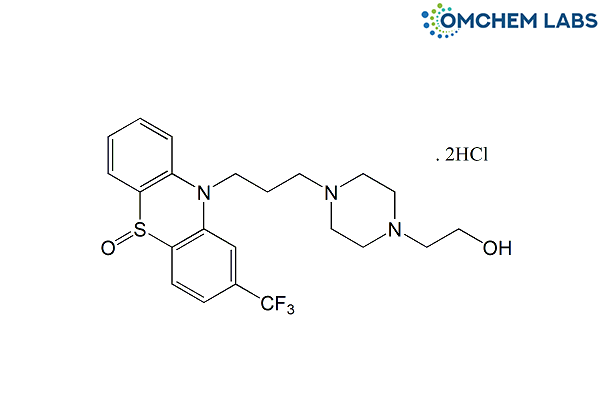
Fluphenazine Dihydrochloride Impurity A / Fluphenazine Decanoate Impurity A
| Catalogue No |
FLUP-OCL-003 |
| CAS NO |
1674-76-6 |
| Molecular Formula | C22H28Cl2F3N3O2S |
| Molecular weight | 526.44 |
| Inquiry Status | In Stock |
| Synonyms | 4-[3-[5-Oxido-2-(trifluoromethyl)-10H-phenothiazin-10-yl]propyl]-1-piperazineethanol dihydrochloride |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
A General Overview of Fluphenazine Decanoate Impurity A: Chemical and Regulatory Perspectives
Fluphenazine Decanoate Impurity A is one of the known impurities associated with the synthesis or degradation of Fluphenazine Decanoate, a typical antipsychotic drug used in long-term management of schizophrenia. This article provides a general overview of the impurity from a pharmaceutical and regulatory standpoint. Without delving into specific quantitative data, the discussion centers around the significance of impurity profiling, regulatory expectations, and general characteristics of Impurity A within the framework of pharmaceutical quality control.
1. Introduction
Fluphenazine Decanoate is a depot formulation of Fluphenazine, a phenothiazine derivative, widely used in psychiatric medicine. Like all pharmaceutical agents, its manufacturing and storage processes may lead to the presence of trace impurities. Among these, Impurity A is recognized as a related substance requiring monitoring under international pharmacopeial standards. Understanding and controlling impurities like this one is vital to ensure patient safety and drug efficacy.
2. Origin and Formation
Impurity A in Fluphenazine Decanoate is generally understood to arise from synthesis-related byproducts or from degradation under certain storage or environmental conditions. It may also result from the presence of intermediates or precursors used during the formulation process. Though structurally related to the parent compound, its pharmacological activity and toxicological potential necessitate its identification and limited presence.
3. Regulatory Context
Global regulatory agencies such as the ICH (International Council for Harmonisation), US FDA, and EMA mandate strict guidelines for the identification, qualification, and quantification of impurities. Impurity A is typically listed as a specified impurity, meaning its presence must be controlled below an established threshold. While specific limits and testing methodologies vary, the importance of impurity profiling in maintaining drug safety is universally acknowledged.
4. Analytical Considerations
Standard pharmaceutical practices require the development of robust analytical methods, such as HPLC or GC, for the detection of related substances like Impurity A. Although exact methods and validation parameters are product-specific, the general goal is to ensure accuracy, precision, and reproducibility in monitoring impurity levels throughout a product’s shelf life.
5. Conclusion
Fluphenazine Decanoate Impurity A represents a standard challenge in pharmaceutical quality assurance. While not necessarily harmful in trace amounts, its monitoring exemplifies the industry’s commitment to drug safety and regulatory compliance. A broad understanding of its formation, regulatory status, and analytical monitoring is essential for pharmaceutical professionals engaged in the development and quality control of long-acting injectable medications.
Keywords: Fluphenazine Decanoate, Impurity A, pharmaceutical impurities, drug safety, regulatory compliance, impurity profiling
