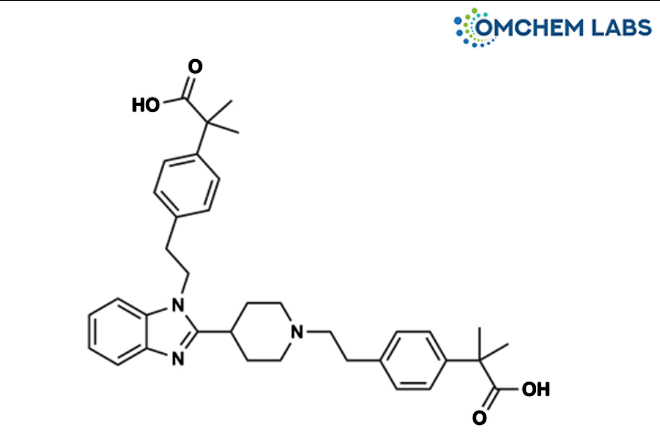
Bilastine Impurity 16
| Catalogue No |
BILA-OCL-001 |
| CAS NO |
2411093-91-7 |
| Molecular Formula | C36H43N3O4 |
| Molecular weight | 581.80 |
| Inquiry Status | In Stock |
| Synonyms | 2-(4-(2-(4-(1-(4-(2-carboxypropan-2-yl)phenethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)-2-methylpropanoic acid |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Bilastine Impurity 16: Scientific Perspectives on Characterization and Control
Introduction
The comprehensive understanding and control of impurities in pharmaceutical substances are fundamental to ensuring product quality, patient safety, and regulatory compliance. Among these, Bilastine Impurity 16 presents an important target for detailed impurity profiling. As pharmaceutical synthesis becomes increasingly complex, so too does the need for robust methodologies that can characterize structurally related compounds with accuracy. Impurity profiling not only enhances the understanding of the API’s chemical stability and reactivity but also supports the development of well-defined specifications and purification strategies throughout the product lifecycle.
Formation of Impurities During API Synthesis
The synthetic pathways employed in the manufacture of Bilastine Impurity 16—as with other pharmaceutical intermediates—often involve multistep reactions, each of which can introduce impurities through incomplete reactions, side reactions, or environmental degradation. Residual reagents, catalysts, solvents, and starting materials may persist in trace amounts. Moreover, reaction conditions such as pH shifts, temperature extremes, or prolonged processing can catalyze the formation of unwanted by-products. In some cases, impurities emerge not directly from synthesis, but as transformation or degradation products due to light, moisture, or storage instability. Thus, understanding the chemical context in which such impurities arise is essential for their control and mitigation.
Analytical Data Interpretation Techniques
Accurate identification and structural elucidation of Bilastine Impurity 16 relies on a suite of analytical methods capable of resolving chemically similar entities. Chromatographic separation—most notably through HPLC or GC—serves as a preliminary tool for isolating the impurity of interest. Coupling these with mass spectrometry allows for high-resolution mass data, aiding in molecular weight determination and fragmentation pattern analysis. Nuclear Magnetic Resonance (NMR) spectroscopy further contributes detailed insights into structural conformation and functional group connectivity. Interpretation of these datasets requires a comprehensive analytical framework, where multiple orthogonal techniques are used synergistically to confirm identity and assess purity.
Method Validation for Impurity Detection
Any analytical procedure intended to detect and quantify Bilastine Impurity 16 must undergo thorough validation to ensure reliability and reproducibility across different laboratories and production lots. The validation process includes evaluating the method's specificity—its ability to distinguish the impurity from the main compound and other matrix components—as well as precision, accuracy, and sensitivity. Parameters such as the detection limit and quantitation limit play a central role in determining whether the method is suitable for trace-level monitoring. A validated method becomes a cornerstone for routine quality control and regulatory acceptance, enabling consistent monitoring of impurity levels over time.
Purification Strategies for Reducing Impurities
Once identified, strategies for reducing or eliminating Bilastine Impurity 16 must be tailored to its physical and chemical characteristics. Common purification approaches include recrystallization, which exploits solubility differences; liquid-liquid extraction, where partition coefficients guide selective separation; and chromatographic techniques such as flash or preparative HPLC for high-purity isolations. The choice of solvent systems, temperature control, and purification cycles can significantly influence the yield and purity of the API. Effective purification does not merely improve visual or assay purity, but also ensures that trace-level impurities remain within acceptable thresholds.
Isolation and Characterization of Impurities
When an impurity exceeds qualification thresholds or remains structurally ambiguous, isolation is a critical next step. For Bilastine Impurity 16, preparative chromatography or fractional crystallization is often used to obtain sufficient material for characterization. Following isolation, spectroscopic techniques such as NMR, MS, and FTIR are used to confirm the structure and assess any functional similarities to the API or other known degradation products. These data inform toxicological assessments and help in the development of impurity reference standards. The characterization process is not only a regulatory necessity but also a valuable source of knowledge about the compound’s chemical behavior.
Conclusion
The impurity profiling of Bilastine Impurity 16 exemplifies the scientific rigor required in modern pharmaceutical development. Each stage—from impurity formation through analytical evaluation, method validation, purification, and structural elucidation—contributes to a deeper understanding of the API’s integrity and reliability. This integrated approach ensures that impurities are not only detected and quantified but also thoroughly understood and controlled. In doing so, the overall pharmaceutical process becomes more robust, transparent, and aligned with global quality expectations.
