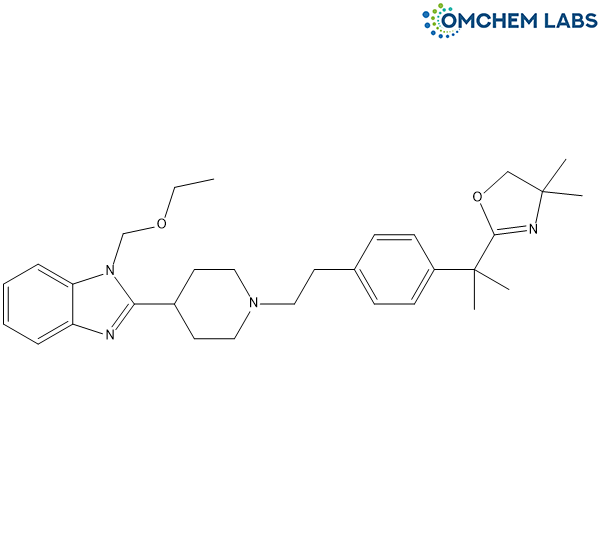
Bilastine Impurity D
| Catalogue No |
BILA-OCL-003 |
| CAS NO |
202189-77-3 |
| Molecular Formula | C32H44N4O2 |
| Molecular weight | 516.73 |
| Inquiry Status | In Stock |
| Synonyms | 2-(2-(4-(2-(4-(1-(2-ethoxyethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)propan-2-yl)-4, 4-dimethyl-4, 5-dihydrooxazole |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Bilastine Impurity D: A Scientific Overview
Introduction
Impurity profiling plays a pivotal role in the pharmaceutical industry, particularly in ensuring the quality, safety, and efficacy of active pharmaceutical ingredients (APIs). Bilastine Impurity D, like many pharmaceutical impurities, arises as an inevitable by-product during the synthesis and handling of the parent compound. Understanding the nature, formation, and control of such impurities is essential to comply with stringent regulatory requirements and to maintain therapeutic consistency. This paper presents a comprehensive overview of the impurity profiling process for Bilastine Impurity D, emphasizing the scientific principles underlying its formation, detection, validation, and control.
Formation of Impurities During API Synthesis
The genesis of impurities such as Bilastine Impurity D typically occurs throughout the synthetic pathway of the API. Various factors contribute to impurity formation, including incomplete reactions, side reactions, reagent or catalyst degradation, and environmental influences such as moisture and temperature fluctuations. Process parameters like reaction time, pH, and solvent choice can also impact the impurity profile. Additionally, impurities may form post-synthesis due to chemical instability, exposure to light or oxygen, and storage conditions. A thorough understanding of these variables is crucial for anticipating potential impurities and designing processes to minimize their generation.
Analytical Data Interpretation Techniques
Characterizing Bilastine Impurity D demands the application of advanced analytical technologies capable of sensitive and selective detection. Techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), liquid chromatography coupled with mass spectrometry (LC-MS), and nuclear magnetic resonance (NMR) spectroscopy are commonly employed. Each method contributes distinct and complementary data, enabling detailed elucidation of impurity structure and concentration. The integration of chromatographic separation with spectrometric identification facilitates comprehensive impurity profiling, aiding in the distinction between known and unknown contaminants.
Method Validation for Impurity Detection
Robust validation of analytical methods is fundamental to ensuring that impurity detection is accurate, reproducible, and fit for purpose. Validation parameters include specificity to distinguish Bilastine Impurity D from other components, sensitivity to detect low-level impurities, linearity across concentration ranges, precision for consistent results, and accuracy to reflect true impurity levels. Adherence to internationally recognized guidelines such as ICH Q2(R1) is essential for establishing method reliability, which is critical for regulatory acceptance and quality assurance.
Purification Strategies for Reducing Impurities
Effective purification techniques are employed to reduce the levels of impurities such as Bilastine Impurity D in the final API product. Common approaches include crystallization, which exploits differences in solubility; chromatographic methods, which separate based on chemical affinity; and solvent extraction, which partitions impurities from the desired compound. The choice of purification strategy depends on the physicochemical properties of the impurity and the API, aiming to achieve optimal purity without compromising yield or product integrity.
Isolation and Characterization of Impurities
When impurities surpass defined thresholds or require further evaluation, isolation and detailed characterization become imperative. Preparative chromatographic techniques allow for the separation of Bilastine Impurity D from complex mixtures. Subsequent structural elucidation is achieved through spectroscopic methods including NMR, mass spectrometry, and infrared spectroscopy. This comprehensive characterization informs toxicity assessment, regulatory documentation, and supports the development of impurity reference standards for ongoing quality control.
Conclusion
The impurity profiling of Bilastine Impurity D encompasses an integrated approach involving knowledge of synthetic chemistry, analytical sciences, and process optimization. Identifying and controlling impurities ensures compliance with regulatory standards while safeguarding patient safety. By combining robust analytical techniques, validated detection methods, strategic purification, and thorough impurity characterization, pharmaceutical scientists can maintain the quality and consistency of Bilastine products. This holistic impurity management approach is indispensable for the successful lifecycle management of APIs and their formulations.
