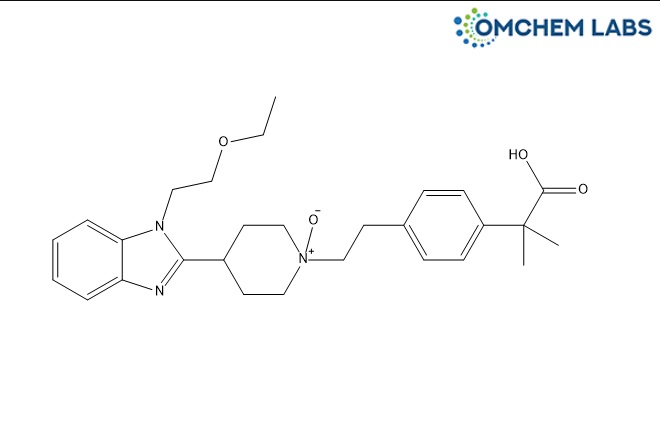
Bilastine N-Oxide
| Catalogue No |
BILA-OCL-002 |
| CAS NO |
2069238-47-5 |
| Molecular Formula | C28H37N3O4 |
| Molecular weight | 479.62 |
| Inquiry Status | In Stock |
| Synonyms | 1-(4-(2-Carboxypropan-2-yl)phenethyl)-4-(1-(2-ethoxyethyl)-1H-benzo[d]imidazol-2-yl)piperidine 1-Oxide, Bilastine N-Oxide |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Bilastine N-Oxide: A Comprehensive Overview
Introduction
Impurity profiling represents a critical facet of pharmaceutical quality control, ensuring the safety, efficacy, and regulatory compliance of active pharmaceutical ingredients (APIs). Bilastine N-Oxide, a metabolite and degradation product of Bilastine, requires thorough investigation to understand its impurity profile. Identifying and controlling impurities in pharmaceutical substances like Bilastine N-Oxide is essential to uphold product quality and patient safety throughout the drug’s lifecycle. This overview discusses the formation, detection, validation, purification, and characterization of impurities related to Bilastine N-Oxide.
Formation of Impurities During API Synthesis
The generation of impurities during the synthesis of Bilastine N-Oxide can arise from various chemical and environmental factors. Impurities may originate from unreacted starting materials, side reactions, incomplete conversions, or degradation processes occurring under stress conditions such as temperature, humidity, or light exposure. Additionally, synthetic intermediates or residual solvents may contribute to the impurity profile. Understanding the reaction pathway and process conditions is vital to anticipate and minimize impurity formation, thereby enhancing the purity of the final product.
Analytical Data Interpretation Techniques
Detecting and identifying impurities within Bilastine N-Oxide involves sophisticated analytical methods. Chromatographic techniques including high-performance liquid chromatography (HPLC) and gas chromatography (GC) are routinely used for separation and quantification. Coupling these methods with mass spectrometry (MS) or nuclear magnetic resonance (NMR) spectroscopy allows for structural elucidation and confirmation of impurities. Analytical data interpretation involves careful examination of chromatograms, spectra, and mass fragmentation patterns to distinguish impurities from the API and understand their chemical nature.
Method Validation for Impurity Detection
Ensuring the accuracy and reliability of analytical techniques for impurity detection necessitates comprehensive method validation. Validation activities verify that the chosen methods provide reproducible, precise, and specific results aligned with regulatory guidelines such as ICH Q2(R1). Key parameters including accuracy, precision, specificity, linearity, sensitivity, and robustness are assessed. A validated method guarantees confidence in impurity data, supporting quality control and regulatory submission processes.
Purification Strategies for Reducing Impurities
Effective purification is crucial to reduce impurity levels in Bilastine N-Oxide to acceptable limits. Various techniques such as recrystallization, solvent extraction, distillation, and preparative chromatography can be employed based on the chemical properties of impurities and the API. The choice of purification method aims to selectively remove impurities without compromising yield or product integrity. Implementing robust purification protocols contributes significantly to the overall quality of the pharmaceutical substance.
Isolation and Characterization of Impurities
When impurities exceed threshold levels or are structurally unknown, isolation and detailed characterization become necessary. Isolation is typically achieved via preparative chromatographic techniques, allowing for the separation of individual impurities. Subsequent characterization using spectroscopic methods such as NMR, MS, and infrared (IR) spectroscopy provides insight into molecular structure and potential pharmacological or toxicological relevance. This information aids in the establishment of impurity limits and informs risk assessment.
Conclusion
The impurity profiling of Bilastine N-Oxide encompasses a multidisciplinary approach integrating synthesis understanding, advanced analytical techniques, stringent method validation, targeted purification, and comprehensive impurity characterization. This holistic process ensures that impurities are controlled effectively, thereby maintaining the safety, efficacy, and regulatory compliance of the pharmaceutical product. Developing a tailored impurity management strategy for Bilastine N-Oxide exemplifies the broader commitment to pharmaceutical quality and patient safety.
