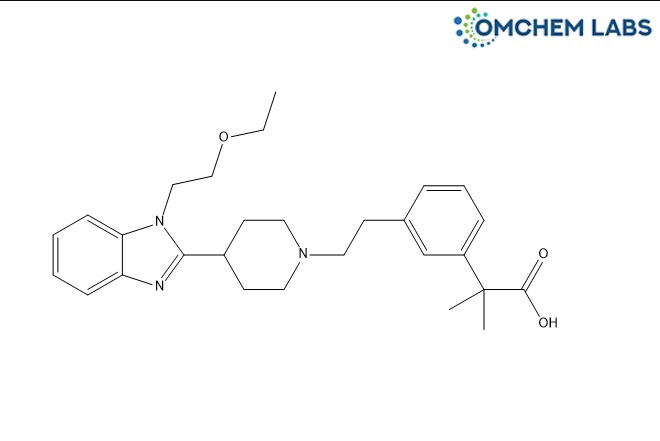
Bilastine Impurity 12
| Catalogue No |
BILA-OCL-005 |
| CAS NO |
1215537-40-8 |
| Molecular Formula | C28H37N3O3 |
| Molecular weight | 463.60 |
| Inquiry Status | Out of Stock |
| Synonyms | Bilastine Impurity 12 Bilastine Impurity F 2-(3-(2-(4-(1-(2-Ethoxyethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)-2-methylpropanoic acid |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Bilastine Impurity 12: A Holistic Approach to Structural Clarity and Quality Assurance
Introduction
The presence of impurities in pharmaceutical substances is an inevitable outcome of chemical synthesis and process variables. In the case of Bilastine Impurity 12, thorough understanding and control of impurity formation are essential to ensure the safety and efficacy of the final drug product. Regulatory bodies emphasize the identification and qualification of impurities even at trace levels, as they may influence therapeutic outcomes or patient safety. Impurity profiling is therefore not just a regulatory obligation but a cornerstone of pharmaceutical quality control and product development. Addressing Bilastine Impurity 12 requires a strategic balance of analytical insight, process refinement, and methodical documentation.
Formation of Impurities During API Synthesis
Impurities such as Bilastine Impurity 12 often originate from predictable chemical interactions or unforeseen side reactions that take place during the synthesis of the active pharmaceutical ingredient (API). These may stem from unreacted starting materials, incomplete conversions, or by-products generated through parallel reaction pathways. Other contributing factors include residual catalysts, process intermediates, and degradation under storage or processing conditions. Environmental influences such as pH shifts, exposure to light, and thermal instability may also lead to the transformation of the API or intermediates into secondary impurities. Thus, a detailed process map is essential for anticipating and limiting such unintended formations.
Analytical Data Interpretation Techniques
For a compound like Bilastine Impurity 12, accurate impurity profiling depends heavily on advanced analytical technologies and data interpretation skills. Chromatographic techniques—including high-performance liquid chromatography (HPLC) and ultra-performance liquid chromatography (UPLC)—are employed to separate and visualize the impurity profile with high resolution. These are typically coupled with mass spectrometry (MS) or nuclear magnetic resonance (NMR) spectroscopy for structural elucidation. Sophisticated interpretation of the resulting spectral or chromatographic data enables scientists to distinguish minor impurities from the main compound, trace their origin, and assess their structural integrity. Interpretation also supports trend analysis during scale-up and long-term stability monitoring.
Method Validation for Impurity Detection
Once a reliable method has been developed for detecting Bilastine Impurity 12, it must be validated to demonstrate its suitability for routine use. Method validation ensures the analytical procedure is capable of consistently producing accurate and precise results under defined conditions. Key parameters such as specificity, linearity, accuracy, sensitivity, robustness, and system suitability are evaluated according to international regulatory frameworks. By confirming the method’s reproducibility and sensitivity, validation provides confidence that even low-level impurities can be reliably monitored, which is essential for ensuring batch-to-batch consistency and regulatory compliance.
Purification Strategies for Reducing Impurities
Controlling the levels of Bilastine Impurity 12 within acceptable limits often requires tailored purification strategies. Depending on the chemical nature of the impurity and its physicochemical differences from the API, a variety of separation techniques may be considered. Crystallization is frequently used for solid APIs where solubility profiles vary significantly. Other approaches include solvent extraction, distillation, and various forms of chromatographic separation such as flash or preparative HPLC. Selection of the optimal purification technique depends on impurity behavior, cost-effectiveness, and scalability. Continuous refinement of these techniques supports not only impurity reduction but also overall yield optimization.
Isolation and Characterization of Impurities
When an unknown or unqualified impurity such as Bilastine Impurity 12 is detected above a threshold level, it must be isolated and characterized to determine its structure and potential toxicity. Isolation typically involves multiple rounds of chromatographic separation until the impurity is obtained in a sufficiently pure form. Structural characterization is then carried out using advanced spectroscopic techniques like NMR, MS, and infrared (IR) spectroscopy. Through this, detailed information about molecular structure, functional groups, and stereochemistry is obtained. The resulting structural insight allows for a toxicological risk assessment and supports the creation of reference standards for future monitoring.
Conclusion
Profiling of Bilastine Impurity 12 embodies the pharmaceutical industry's commitment to quality, safety, and scientific rigor. From understanding its formation to validating analytical methods, purifying the product, and fully characterizing the impurity, each phase contributes to a comprehensive quality control framework. This multi-faceted approach ensures regulatory compliance while upholding therapeutic integrity. As drug development advances and regulatory expectations become more precise, a proactive impurity management strategy remains vital in safeguarding both product quality and patient trust.
