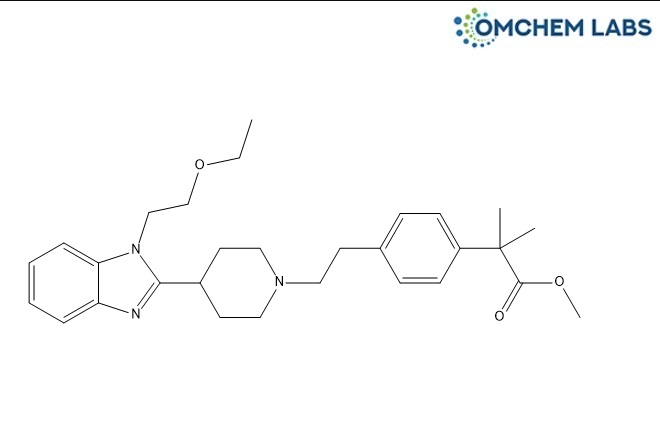
Bilastine Methyl Ester
| Catalogue No |
BILA-OCL-007 |
| CAS NO |
1181267-38-8 |
| Molecular Formula | C29H39N3O3 |
| Molecular weight | 477.60 |
| Inquiry Status | In Stock |
| Synonyms | Bilastine Methyl Ester, Methyl 2-(4-(2-(4-(1-(2-ethoxyethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)-2-methylpropanoate |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Bilastine Methyl Ester: A Scientific Overview
Introduction
Impurity profiling is a critical component in the pharmaceutical development and manufacturing process of active pharmaceutical ingredients (APIs) such as Bilastine Methyl Ester. The presence of impurities, regardless of their source, can influence the quality, safety, and efficacy of the pharmaceutical product. Comprehensive understanding and control of these impurities are necessary to meet stringent regulatory guidelines and ensure patient safety. This article presents an overview of the general aspects of impurity formation, detection, and control strategies for Bilastine Methyl Ester, providing insights applicable across diverse pharmaceutical compounds.
Formation of Impurities During API Synthesis
Impurities in Bilastine Methyl Ester predominantly arise during the synthetic pathway of the molecule. These impurities may originate from incomplete reactions, side reactions, degradation of intermediates, or contamination from reagents and solvents. Reaction conditions such as temperature, pH, catalyst presence, and reaction time significantly affect the impurity profile. Moreover, storage and handling conditions post-synthesis can introduce degradation impurities. Understanding the synthetic route and process parameters is essential for anticipating potential impurities and designing effective control strategies.
Analytical Data Interpretation Techniques
The identification and quantification of impurities require sophisticated analytical methods capable of distinguishing structurally similar compounds and trace-level contaminants. Techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), liquid chromatography–mass spectrometry (LC-MS), and nuclear magnetic resonance (NMR) spectroscopy are widely utilized. These methods generate detailed chromatographic and spectral data that must be carefully interpreted to identify impurity structures, estimate concentrations, and assess their potential impact on product quality. Effective data interpretation demands expertise in analytical chemistry and knowledge of the molecule’s chemical behavior.
Method Validation for Impurity Detection
Ensuring the reliability of impurity detection methods is paramount and achieved through comprehensive method validation. Validation confirms that the analytical techniques used are suitable for their intended purpose and can consistently deliver accurate and reproducible results. Parameters such as specificity, accuracy, precision, linearity, limit of detection (LOD), and limit of quantification (LOQ) are evaluated during validation. Adherence to international guidelines, including ICH Q2(R1), ensures that impurity profiling methods meet regulatory expectations and support quality control throughout the product lifecycle.
Purification Strategies for Reducing Impurities
Once impurities are identified, appropriate purification methods are employed to minimize their presence in the final API. For Bilastine Methyl Ester, purification approaches can include crystallization, solvent extraction, distillation, and preparative chromatography, among others. The choice of technique depends on the physicochemical properties of both the API and its impurities. Effective purification enhances product purity, reduces the risk of adverse effects, and improves overall process efficiency, contributing to a high-quality pharmaceutical product.
Isolation and Characterization of Impurities
Isolating impurities above regulatory thresholds is crucial for thorough characterization. Isolation is commonly achieved using preparative-scale chromatographic techniques. Characterization involves employing spectral analysis tools such as NMR, mass spectrometry, and infrared spectroscopy to elucidate molecular structures and functional groups. This detailed characterization supports toxicological evaluation, helps establish impurity limits, and informs further refinement of synthetic and purification processes to mitigate impurity formation.
Conclusion
Impurity profiling of Bilastine Methyl Ester is an integral part of ensuring pharmaceutical quality and regulatory compliance. By understanding the formation mechanisms, applying advanced analytical techniques, validating detection methods, optimizing purification, and thoroughly characterizing impurities, manufacturers can maintain the safety and efficacy of the final product. The principles outlined here provide a framework adaptable to various APIs, enabling robust impurity management strategies across the pharmaceutical industry.
